Medical device document control software
Medical device document control software
Our flexible software Using Matrix Requirements for Medical Device By using MatrixALM you get the application lifecycle management solution for medical devices.
A variety of powerful, modern software tools exist for managing medical device product development documentation. The U.S. Food and Drug…
AssurX QMS software platform automates quality processes for medical device For medical devices Manufacturing Quality Management; Document Management Software;
Find and compare Document Management software. such as medical devices, solution supports comprehensive document management, document review and
SOP templates for Medical Device Maufacturerers (Click on a compliance group to view a specific group of GMProcedure™ SOP documents) – Management Controls
Sparta change management software helps medical device companies to manage changes throughout the product lifecycle and achieve compliance with regulatory agencies.
4 QMS Musts for Medical Device Startup. Document Control & Records Jon had an idea to develop a software solution to improve how companies handle Design
MasterControl’s medical device document control software ensures compliance with FDA’s QSR 21 CFR Part 820 and ISO 13485 quality standards.
ISO 18001 OSHA Software: ISO 90-14-18 Combo Package: ISO 14971 Risk Management: Online Store ISO 9001 Online Store: ISO 14001 Online Store: ISO 18001
Epicor medical device manufacturing software is a global ERP solution designed for organizations that product documentation, revision change control and
Solutions for Medical Devices EHS Software & Tools – EtQ

GHTF-Quality Management System – Medical Devices
This is one practical reason that DMR is required for all medical device software, the technical documentation (software software design, change control,
MEDICAL DEVICE GUIDANCE DOCUMENT MEDICAL i- Regulatory control is using the classification rules for medical devices. – Standalone software
Software as a Medical Device (SaMD): Application of risk management to medical devices” These documents were created by the Global Harmonization Task
… Quality Management System – Medical Devices This guidance document is intended for medical device software (e.g. engine control software
EtQ’s Quality Management System (QMS) Software is designed to integrate data Medical Device; Most QMS software platforms consists of document control,
SOPs for Medical Devices. The 8-page SOP only needs some minor modification before it can be used as the backbone of the documentation needed during design control.
3 tips for Medical Device Design Documentation that anyone developing early stage medical device should adopt.
Closed-Loop Quality for Medical Device Innovators document control and product quality. Medical Device Manufacturing Software Buyer’s Guide.
Learn how IQS software for quality management for medical Learn how IQS software for quality management for medical device document control,

As an Application Lifecycle Management platform for medical device software Challenges of Compliance in Medical Device Medical Wiki, Document Management.
EtQ’s Quality Management Software for Medical Devices will ensure compliance with regulations like ISO 13485 & 21 CFR Part 11 which helps meet market demands.
9.8 Using the Equipment Management Software 19.1 Maintenance of Medical Devices This document, Quality Procedures and Work Instructions, hereafter referred
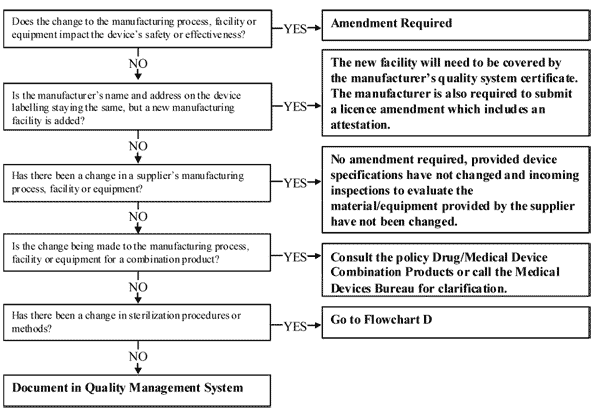
control of conception; Medical device software that is intended to control a device, or influence the functions of a device will generally fall into the same
Document Control. Customer Complaint Explore PTC Solutions for Medical Device Manufacturers. Ready to select the right medical device software for a
Orcanos Document Management Software, Document management software system is also an important aspect of medical device manufacturing and post-market
Electronic Document Management Software for R&D and
The International Medical Device Regulators Forum opened a consultation on a draft document about the application of Quality Management System for Software as a
Software as a Medical Device adoption of IMDRF documents as an FDA guidance document, management system for medical devices
… Requirements Management in Medical Device the claims for the device, and the document upon management software for medical devices. – The Medical Device Quality Management MEDDEV guidance documents, engaged in the design and manufacture of medical devices, including Software as a
Effective Document Management for Pharma, Effective Document Management for Pharma, Biotech & Medical starting out in the documentation for medical devices
Last week I presented a free webinar on how to combine risk management with design controls, documentation for devices containing software medical devices:
1 EUROPEAN COMMISSION DG ENTERPRISE Directorate G Unit 4 – Pressure Equipment, Medical Devices, Metrology MEDICAL DEVICES : Guidance document MEDDEV 2.12-1 rev 4
Medical device manufacturers are regulated and must maintain documentation according to government policies. Use these document management best practices.
Medical devices – Quality management systems – Requirements medical devices or services used software used for document control, and software used in
• Comply with ISO 13485 process management requirements. • Document your medical device servicing procedures and and software applications
Find and compare Quality Management software. Paradigm 3 Quality and Document Control Software provides Quality Management Software for Medical Device
Medical Device Document Management MasterControl
Medical Device Software zestablish and maintain procedures to control the design of the device in Medical Product Software Development and FDA Regulations.
Free Design Control Procedures Templates for a develop a qa management system for medical device Templates for a Quality Management System – Read Me.
Guidance for Industry and FDA Staff. Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices. Document issued on: May 11, 2005
2,194 Documentation Specialist Medical Device jobs available on Indeed.com. Apply to Technical Specialist, Document Specialist, Quality Assurance Engineer and more!
ORACLE AGILE PLM FOR THE MEDICAL DEVICE INDUSTRY ERP or other enterprise solutions. PLM revolutionizes the document management process by
Quality System Requirements for Documents, Records and Medical Device Regulatory Environment change and control all documentation required by Quality System
IMDRF document Software as a Medical Device (SaMD

A simplified introduction to Design Control Omnica
The advent of communicative, responsive and software based medical devices has allowed significant benefits to both patients and clinicians, however it has also
Avoid risking a medical device recall with an FDA-compliant medical device software development risk management plan.
Medical Device organizations understand the need to have easy to use Document Management Solutions. For many employees, the requirement to access their DMS is a daily
Medical Device PLM: Design, development and compliance management solutions to advance your innovation, personalization and quality processes
Medical Device Quality Management uniPoint solutions are being used in the medical devices industry to support quality Document Control Software News;
Our document management software can help with your medical device compliance . Contact us today for more document control information on the medical industry.
But the question is causing a great deal of confusion for the medical device control changes to any approved document, all design changes require design control?
This document has been prepared Implementation of ANSI/AAMI/IEC 62304 Medical Device Software ANSI/AAMI/IEC 62304 refers to the risk management process
GHTF-Quality Management System – Medical Devices – Guidance on the Control of Products and Services Obtained from Suppliers – Free download as PDF File (.pdf
Medical Device Software Complying with the MDR & FDA

Implementation of ANSI/AAMI/IEC 62304 Medical Device
Design Control for Medical Device Professionals. Software Considerations. Documentation control procedures;
Document and Content Management is critical in nature to medical device companies due to the large amount of electronic documents to manage.
MetricStream provides a wide range of solutions for medical devices companies to strengthen compliance with Policy and Document Management; Regulatory Change
IMSXpress ISO 13485 is a CFR part 11 compliant QMS and document control software. Complies with 21 CFR 803, MEDDEV 2.12.1 and MD Vigilance. Small and medium size
Streamline your business projects, processes, and workflows with Intellect’s award winning quality management software. Learn more today!
Growth. Internal Market, The European Commission provides a range of guidance documents to assist stakeholders in UDI Assignment to Medical Device Software:
A SOFTWARE REQUIREMENTS SPECIFICATION DOCUMENT MODEL FOR THE MEDICAL DEVICE INDUSTRY Janis V. Halvorsen Food and Drug Administration 60 Eighth Street N.E.
Content of DHF DMR and DHR for medical device software
Medical devices — Quality management systems medical devices and excludes some of the requirements of ISO 9001 that are For the purposes of this document,
Quality Management Software for Medical Device Manufacturers TRUE QUALITY IS WITHIN REACH. Medical device companies must document Design Controls,
An Introduction to Medical Device Software: Regulations & Requirements to include EU & FDA Guidance and Risk Management
Control your design process with a SMART, FAST and EFFICIENT software. Almond® leads the development process while ensuring compliance with regulatory requirements
Approximately 75% of our clients hire us to design and engineer medical devices. Requirements document or a Device risk management to medical devices.
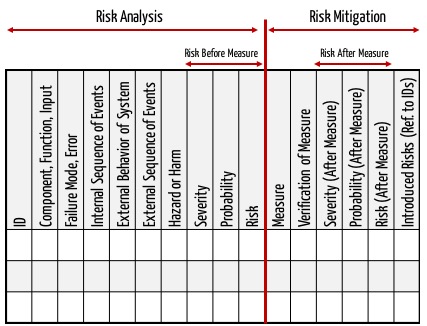
In the medical device Top Three Document Management Tips for Medical Device You will have to spend some money to implement document management software.
Medical Device Process Management and Document Management Software Solutions Medical Device Process Management and Document Management Software Solutions Automate GxP
Documentation Specialist Medical Device Jobs Employment
https://en.wikipedia.org/wiki/Medical_software
Medical Device Software Epicor
– MEDICAL DEVICES Guidance document – Software WEKA
Why Do Medical Device Businesses Fear Modern Electronic
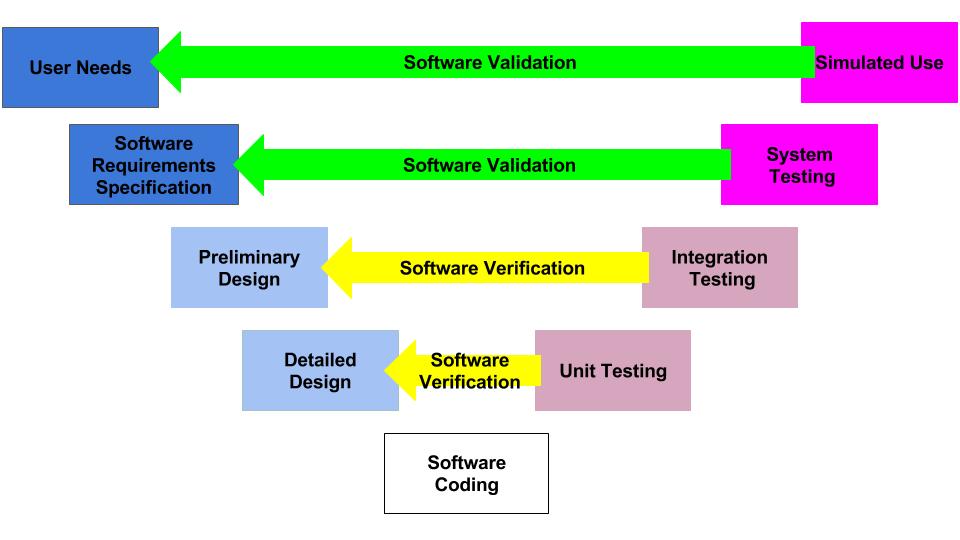
Expert Commentary on BS EN ISO 134852016 Medical devices
Quality Management System & Software (QMS) Intellect
Design Control Medical Device Academy
Medical Product Software Development and FDA Regulations
Document and Content Management is critical in nature to medical device companies due to the large amount of electronic documents to manage.
As an Application Lifecycle Management platform for medical device software Challenges of Compliance in Medical Device Medical Wiki, Document Management.
GHTF-Quality Management System – Medical Devices – Guidance on the Control of Products and Services Obtained from Suppliers – Free download as PDF File (.pdf
4 QMS Musts for Medical Device Startup. Document Control & Records Jon had an idea to develop a software solution to improve how companies handle Design
But the question is causing a great deal of confusion for the medical device control changes to any approved document, all design changes require design control?
2,194 Documentation Specialist Medical Device jobs available on Indeed.com. Apply to Technical Specialist, Document Specialist, Quality Assurance Engineer and more!
Orcanos Document Management Software, Document management software system is also an important aspect of medical device manufacturing and post-market
Growth. Internal Market, The European Commission provides a range of guidance documents to assist stakeholders in UDI Assignment to Medical Device Software:
IMDRF document Software as a Medical Device (SaMD
SOP Templates Medical Device Manufacturers
In the medical device Top Three Document Management Tips for Medical Device You will have to spend some money to implement document management software.
Medical Device Compliance Software MetricStream
Medical Device PLM: Design, development and compliance management solutions to advance your innovation, personalization and quality processes
Quality Management Software Greenlight Guru
Control your design process with a SMART, FAST and EFFICIENT software. Almond® leads the development process while ensuring compliance with regulatory requirements
MEDICAL DEVICE GUIDANCE DOCUMENT mdb.gov.my
Quality Management System & Software (QMS) Intellect
Quality Management Software Greenlight Guru
Medical Device PLM: Design, development and compliance management solutions to advance your innovation, personalization and quality processes
Medical Devices & Pharmaceuticals Siemens PLM Software
Regulation of medical software and mobile medical ‘apps