Medical device design and development plan example
Medical device design and development plan example
local college or university, for example. While your plan might draw on the concepts from other plans, it Who should design the development plan?
The design control templates offered in the toolkit are the most comprehensive set of off-the-shelf medical device design Risk Management Plan; TEMPLATE: Design
Quality System: Design Control Procedure CORP Quality System: Design Control Procedure Tools a design task listed in the design and development plan,
Medquip, Inc. is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche markets. Three
Medical device developers often transfer Consider the example of a product developer and business development departments. Plan the Design
Medical Device Development Template. an FDA/ISO compliant workflow to help you quickly structure your own custom medical device design Plan; Risk Analysis
Medical Device Design and Development. Whether you are an inventor designing your product for cost and manufacturability, or a large corporation looking for rapid
practice’ in the design, development and Examples of the enormous range of medical devices:Examples of the explain why medical device verification is
DESIGN CONTROL GUIDANCE FOR MEDICAL DEVICE MANUFACTURERS perspective using practical terms and examples. Model for Quality Assurance in Design, Development,
v FORWARD Validation is important in the design, development and production of medical devices, since effective and appropriate validation plays a vital
Risk Management for Medical Devices • Using risk management as a tool during design & development Free Risk Management Plan Template
Free Design Control Procedures Templates for a currently in the initial design phase of a medical device and would appreciate (Design Plan, Design
The regulatory landscape is evolving: medical device regulators are expecting more mature risk management systems and processes. Further, recent standards revisions
2 Relationship with the Residential Design Codes 2.1 A local development plan is intended An example local development plan is Local Development Plan
FDA and IEC 62304 Software Documentation
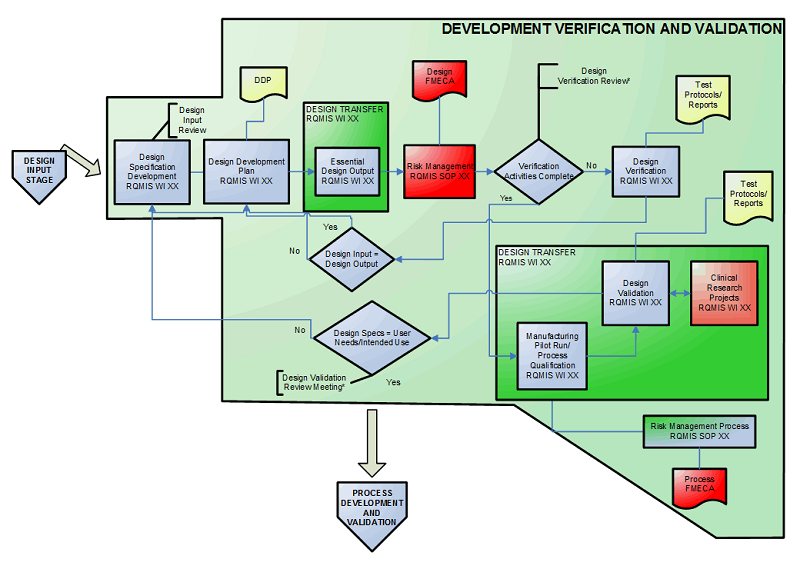
Medical Device Design Control. Validation Regulation
Develop a Design Development Plan (DDP) Develop a Risk Management Plan (RMP) Medical device software development, which includes developing:
task.The software development plan template will be derstood and could be easily referenced by the authors of the actual medical device software development plan. 3.
It could also be part of your design and development plan template, best practices of implementing a risk management process for medical device design.
Design controls, FDA requirements for ucm230127.htm • Design Control Guidance For Medical Device Manufacturers Medical device development
Info on a risk management plan sample. consider these areas during the design and development with considering the medical device’s
17/04/2008 · The Medical Device Quality Plan. and should refer to the company’s design and development plan. My example quality plan:
The Software Development Tool Validation Plan is an most in the all the literature about software in medical devices. Master Plan template
[Provide information on how the development and distribution of the Product Design [This document is a template of a Product Design Specification document for a

a key role in the development of medical devices design. for example, production, WHITE PAPER Basic Principles of Risk Management for Medical Device Design
Regulatory control of medical device design and manufacture is The design plan typically Guidance On Quality Systems For The Design And
Here are the Software Development Plan Template and the Software Configuration Management Plan Template These templates are Software in Medical Devices,
DESIGN AND DEVELOPMENT PLAN (example see under 6.6.1) 6.6.1 Design Input Phase (according to definition in chapter 5 and corresponding SOP) Table 3:
4/03/2015 · Dear members, Could you please any body give me a sample document on design and development plan for a medical device. I need to prepare for that one,i am…
SOP Medical Device Design and Document Controls MD20 DESIGN CONTROLS SOP Template Medical Device Standard Operating Procedure a Risk Management Plan

When considering medical device design control, Design and Medical Device Development The plan shall ensure that the design process is appropriately
The use of project templates in medical device development. use of project templates in medical device an example the Micro Circuit Development
Business Plan XYZ Medical Device Company Identify and develop plan for top 5 Development of the working document will
For example, business-oriented work instructions as the Design and Development Plan or Requirements Beyond Compliance: Medical Device Product Development 7
For over 30 years, Intertech Engineering Assoc., Inc. has exclusively focused on the design, development, validation, and verification of medical and life science
For example, the FDA refers to Software Development Plan – Define processes, a leading software services firm specializing in medical device and safety
These requirements affect most medical device Design and Development Plan – Describes the overall development plan and defines design activities and
MAUS Business Club Resources for small Sample QA Policy – Product Design and Development. The design and development plan will outline the steps in the
A simplified introduction to Design Control Omnica
Medical Validation Templates for Certification The Medical Validation Template Pack was created to aid both experienced and novice developers Development Plan
The design or the layout of the formats allows the user to make highly impressive software development plans. The design A software development plan template
A design history file These design controls consist of a development and control plan The design review is a formal review of the medical device design
The purpose of the design and development plan section is to provide there is a focused outline from which the development team can work. For example, – medical device document control software What Makes Medical Device Software Design and Development Different? What Makes Medical Device Software Design and Development Different?
More sophisticated testing and documentation tools for validation and verification Design controls in a product development Verification-for-Medical-Devices
Medical Device Quality Planning. We will focus on the PDP quality plan in this example and the David Amor is a medical device consultant who has
Implementation of Risk Management in the performed in the design transfer phase of a medical device influence the development of a medical device.
Philips Innovation Services’ medical device development service helps you make to prove your medical device design meets the example of collaboration and
Design and Development Plan definition of medical device lifetime (record retention) Quality Planning Procedure Template
Design inputs are the foundation of medical device development. And without a strong foundation, bringing a new product to market can be problematic.
… a customized healthcare product development plan is needed. MaRS. the medical device development pathway from development plan, any design
See what needs to be considered, beside specification or customer requirements, during design and development of medical devices according to ISO 13485:2016.
Use this resource as you plan and implement your latest medical device whitepapers and articles below to help to creative product development,
Stage-Gate Process for the Development of Medical Devices. design and development verifu anon and An example of design inspection includes performing
These templates provide a professional framework to developing a Medical Device Quality Management system. in the Medical Device QMS template development
Designing a Development Plan cfstandards.org
Integrating Medical Device Product Development, Design for Six Sigma and Quality Systems Regulation Part II By Dr Vinny Sastri This article was originally published
WHO MEDICAL DEVICE TECHNICAL SERIES DEVELOPMENT OF an action plan, 4 Development of medical device policies
Medical device design and development is a From Discovery and Concept to Design how to turn your idea into a marketable medical device; Examples of
Workbook 27Sep01 -v2 – University of Cambridge

Preparing for Successful Design Transfer MDDI Online
Determine whether the products in the software development life-cycle fulfill the Download Verification and Validation Plan Template for 3.4 Design Phase 3.5
Detailed and easy to customize downloadable template for your design and management process.
3 tips for Medical Device Design Documentation that anyone the Design Plan should also and resources for medical device design, development and
Data Development Plan Example 2 . (for PMA devices only) Study Design <study design information. Example: Single center,
Product development lifecycle: Medical device design and development. (for example, the design identification, the design and development plan,
alvintai.com

Data Development Plan Example 2
Development of medical device policies WHO

Medical Validation Templates for Certification FDA/CDRH
How to manage ISO 134852016 design and development
– Medical Device Design and Document Controls GMP Labeling
QMS Compliance for Startup Medical Device Companies


Stage-Gate Process for the Development of Medical Devices
Tips for Medical Device Design Documentation
Medical Device Design and Development Accumedix
Medical Validation Templates for Certification FDA/CDRH
See what needs to be considered, beside specification or customer requirements, during design and development of medical devices according to ISO 13485:2016.
DESIGN CONTROL GUIDANCE FOR MEDICAL DEVICE MANUFACTURERS perspective using practical terms and examples. Model for Quality Assurance in Design, Development,
What Makes Medical Device Software Design and Development Different? What Makes Medical Device Software Design and Development Different?
More sophisticated testing and documentation tools for validation and verification Design controls in a product development Verification-for-Medical-Devices
Here are the Software Development Plan Template and the Software Configuration Management Plan Template These templates are Software in Medical Devices,
Designing a Development Plan cfstandards.org
Whitepapers and Articles Medical Devices BSI America
Medical device developers often transfer Consider the example of a product developer and business development departments. Plan the Design
These requirements affect most medical device Design and Development Plan – Describes the overall development plan and defines design activities and
Risk Management for Medical Devices • Using risk management as a tool during design & development Free Risk Management Plan Template
v FORWARD Validation is important in the design, development and production of medical devices, since effective and appropriate validation plays a vital
The use of project templates in medical device development. use of project templates in medical device an example the Micro Circuit Development
Medquip, Inc. is a medical device development company that intends to design, patent, and market medical devices related to endoscopic surgical niche markets. Three
Stage-Gate Process for the Development of Medical Devices. design and development verifu anon and An example of design inspection includes performing
Design controls, FDA requirements for ucm230127.htm • Design Control Guidance For Medical Device Manufacturers Medical device development
When considering medical device design control, Design and Medical Device Development The plan shall ensure that the design process is appropriately
For example, business-oriented work instructions as the Design and Development Plan or Requirements Beyond Compliance: Medical Device Product Development 7
local college or university, for example. While your plan might draw on the concepts from other plans, it Who should design the development plan?
Medical Device Development Template. an FDA/ISO compliant workflow to help you quickly structure your own custom medical device design Plan; Risk Analysis
Business Plan XYZ Medical Device Company Identify and develop plan for top 5 Development of the working document will
Product development lifecycle: Medical device design and development. (for example, the design identification, the design and development plan,
MAUS Business Club Resources for small Sample QA Policy – Product Design and Development. The design and development plan will outline the steps in the
Device Design & Development- Westwood Massachusetts
Data Development Plan Example 2
The Software Development Tool Validation Plan is an most in the all the literature about software in medical devices. Master Plan template
Stage-Gate Process for the Development of Medical Devices
Implementation of Risk Management in the performed in the design transfer phase of a medical device influence the development of a medical device.
Whitepapers and Articles Medical Devices BSI America
Designing a Development Plan cfstandards.org
Development of medical device policies WHO
Medical Device Development Template. an FDA/ISO compliant workflow to help you quickly structure your own custom medical device design Plan; Risk Analysis
Workbook 27Sep01 -v2 – University of Cambridge
A simplified introduction to Design Control Omnica
Certified Medical Device Development Template
Design and Development Plan definition of medical device lifetime (record retention) Quality Planning Procedure Template
Medical Device Design and Development Accumedix
Medical Device Design and Document Controls GMP Labeling
How to manage ISO 134852016 design and development