Medical device development regulation and law pdf
Medical device development regulation and law pdf
on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices (OJ L 189, 20.7.1990, p. 17).
on medical devices to assess their quality, safety and efficacy.2 Fortunately since the early 1980s, the regulatory paradigm for medical devices has changed exceptionally. 4 Now with the availability of different regulations of the countries or region on medical devices, there is a need to harm-onize regulations in order to curtail regulatory hurdles and expedite access to high quality, safe
David W Feigal, M.D., M.P.H. Senior Vice President for Global Regulation, Pharmacovigilance and Risk Management Élan Pharmaceuticals Regulations for medical
Cambodia, and Laos, they are still under development. However, regulations currently in force do not necessarily imply the manda-tory registration of medical devices for sale, such as in the case of Malaysia. Under the ASEAN Roadmap for Healthcare Integration, medical devices regulations and standards across all member countries, as well as pre-market approval process and post-marketing
41 (1) The Minister may suspend a medical device licence without giving the licensee an opportunity to be heard if it is necessary to do so to prevent injury to the health or safety of patients, users or other persons, by giving the licensee a notice in writing that states the reason for the suspension.
European legislation ensures the safety and efficacy of medical devices and facilitates patients’ access to devices in the European market. To keep up with advances in science and technology, 2 new European Regulations are replacing 3 existing Directives in the years up to 2022.
How the new EU Medical Device Regulation will disrupt and transform the industry 3 1 6 A fundamental impact on innovation 1 8 A c nowledgments 1 9 C ontacts
Medical devices Research and development Regulation Assessment Management PREMARKET APPROVAL WHO MEDICAL DEVICE TECHNICAL SERIES WHO MEDICAL DEVICE TECHNICAL SERIES MEDICAL DEVICE REGULATIONS HEALTH TECHNOLOGY ASSESSMENT OF MEDICAL DEVICES WHO MEDICAL DEVICE TECHNICAL SERIES Assessment Regulation NEEDS ASSESSMENT FOR MEDICAL DEVICES Management MEDICAL DEVICES BY CLINICAL PROCEDURES WHO MEDICAL
• Plan to sample all ‘device subcategory’ and ‘generic device group’ over 5 year certification plan • Technical documentation sampled pre and post market
The European Database for Medical Devices, or Eudamed, serves as a repository for information on medical devices collected by competent authorities and the European Commission. Under current European law, only these parties may access Eudamed.
The REACH Regulation imposes different requirements on medical devices depending on whether they are preparations or articles, and on whether they are manufactured in, or imported into, the EU/EEA.
Learning Objectives This course is specifically focused on the law, regulations and policies set by the FDA for the pre-market approval, manufacture and post-marketing compliance of medical devices.
February 2015. On 26 September 2012, the European Commission published new legislation in the form of two draft Regulations to govern the regulation of medical devices and in vitro diagnostic medical devices in Europe.
medical devices, these tests are stricter as a medical device can be in direct contact with a patient or be located inside a patient, such as a pacemaker. Depending on the type of medical device, there are di erent regulations which
About. The Graduate Certificate in Medical Devices Regulatory Affairs online program presents an in-depth review of regulations relating to the development of medical devices, including those products not specifically regulated by federal law.
– access to safe, effective products of acceptable quality including medical devices – importation for sale/distribution to the public of products, which have prior approval by the Ministry of health
The regulation of medical devices in Europe Role of Standards Mireille De Cré, Pharm D MDCPartners Mireille.decre@mdcpartners.be . 2 Design, trials, manufacturing Regulatory approval CE Mark Market approval / Market access Market feedback: vigilance and surveillance Surveillance audits, Regulatory requests . Medical devices are not what they were 50 years ago Development of medical devices
DEVELOPMENT AND IMPLEMENTATION OF MEDICAL DEVICES

Overview of FDA Regulatory Compliance for Medical Devices
Any medical device company driving new product innovation or changes to existing products in either Class II or Class III categories of products needs to undergo the FDA review process to evaluate the efficacy and product safety.
This Article analyzes the objectives and structure of the 1976 medical device law and concludes that one of the statute’s core values is the prin- ciple of public accountability for Agency actions (Part II).
We are developing guidance for health institutions wishing to apply the exemption to the new in vitro diagnostic medical device regulation (2017/746) and the new medical device regulation (2017/745).
regulation and law, affecting the development and ultimate marketing of new medical products, drugs, devices and biologics. They have served the regulatory needs of large (> billion/year)
medical devices, shown through a combination of education and experience in medical device regulatory affairs. 14 In general, this new position should be an internal position for the manufacturer,
unique device identification system of medical devices in the Union to enhance the traceability of medical devices throughout the whole supply chain contributes to patient safety by facilitating vigilance, market surveillance and transparency in this

The life-cycle of a medical device is calculated from its design and development to manufacture and its subsequent disposal. It can be divided into three common stages, namely pre-market, placement on-market and post-market. Safety and performance of a medical device may affect at each of these stages. It is therefore important that the scope of the regulatory control covers the entire life
ALM for Medical Device Development Compliance with IEC 62304, Title 21 CFR Part 11 (FDA), ISO 14971, IEC 60601 and more As an Application Lifecycle Management platform for medical device software development, codeBeamer ALM provides an efficient way to support and prove the use of mature medical technology development processes.
REGULATORY REQUIREMENTS FOR MEDICAL DEVICESIN THAILAND Yuwadee Patanawong FDA THALAND 5 November 2012 . Products in Control of Thai FDA-Food, Drugs, Psychotropic Substances, Narcotics, Volatile Substances -Medical Devices-Cosmetics-Hazardous substances for household use. Secretary-General 3DeputySecretary Generals Infra-structure of Food and Drug Administration …

Development Testing Manufacture Marketing Transfer Distribution ACQUISITION UTILISATION PROVISION. MEDICAL DEVICE REGULATIONS Global overview and guiding principles WORLD HEALTH ORGANIZATION GENEVA. Acknowledgements This guide was prepared under the principal authorship of Dr Michael Cheng. It is based on a similar publication issued by the Pan American …
The new and expanded Second Edition of Pharmaceutical and Medical Device Law: Regulation of Research, Development, and Marketing, offers readers a comprehensive and readable text about the dynamic and complex area of pharmaceutical and medical device law in …
“medical device family” means a group of medical devices that are made by the same manufacturer, that differ only in shape, colour, flavour or size, that have the same design and manufacturing process and that have the same intended use.
text of the proposed Regulation on medical devices resulting from the negotiations between the Council and the European Parliament. In this revised document the text of Article 1(8a) has been changed and paragraphs 4 and 4a of
• Medical evice Development D Promotion Act was announced on 27 June 2014. 10 (Ref.) Summary of PAL revision • Points the amendment are to; of . 1. Strengthen safety measures regarding drugs and medical devices 2. Revise medical device regulations based on its characteristics 3. Introduce Regenerative and Cellular Therapy Products (RCTP) & Gene Therapy Products (GTP) regulations …
Medical Devices Regulations WIPO
he regulation of medical devices in Europe is cur-rently undergoing a radical overhaul. The current regulatory system is now dated and the rules have not kept up pace with the rapid technological and sci- entific progress that the sector has seen in the last 20 years. In addition, differences in approach by different Member States have led to a lack of harmonisation across Europe. Many also
In November 2014, Japan introduced the Pharmaceutical and Medical Device Act (PMD Act), which made significant changes to the country’s medical device registration process.
MEDICAL DEVICE REGULATIONS IN THE U.S. – THE BASICS This paper is a general summary of food and medical device regulations administered by the U.S. Food and Drug Administration (FDA). There may be other laws or regulations, such as state laws, that affect food and/or medical device products. This paper does not constitute legal advice for any particular situation and does not create … – medical coding tutorial pdf The Medical Device Coordination Group (the MDCG) is an expert group established by Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnostic medical devices. Its members are experts representing competent authorities of the EU countries. The MDCG provides advice and assists the Commission and EU countries in implementation of both Regulations. The …
Mapping the Medical Device Development Process Scott T. Ham Industrial Technology California Polytechnic State University June 4, 2010 . MAPPING MEDICAL DEVICE DEVELOPMENT ii ABSTRACT This project examined the use of process mapping as a tool to show the process of developing medical devices from a broad perspective that includes research, innovation, development, regulation…
of encouraging policies and regulations that the Government has introduced to give a fillip to the medical device industry. For instance, the government has overhauled the regulatory framework for medical device in 2017 and has brought it at par with international norms by introducing the concept of ‘risk-based’ regulation. The regulatory licenses issued for import, manufacture or sale of
Content -Annex 3 –Unannounced inspections 4. High risk devices: sample devices belonging to threedifferent device type and every hundredth type at the end of the production chain or in manufacturer’s warehouse in a view of testing the conformity of the device types.
No. 1] Medical Device Innovation in America 405 This Note proceeds as follows: Part II examines the regulatory environment for premarket medical device review.
Pharmaceutical Administration and Regulations in Japan This file contains information concerning pharmaceutical administration, regulations, and new drug development in Japan updated annually by the English RA Information Task Force, International Affairs Committee, Japan Pharmaceutical Manufacturers Association (JPMA). The contents are not abstracts of governmental rules or regulations …
Regional meetings (1) 1st Regional Meeting of the Regulatory Authorities for the Strengthening of the Regulatory Capacity of Medical Devices in the
The Competent Authorities for Medical Devices (CAMD) Executive Group recommended the establishment of an MDR/IVDR implementation taskforce to facilitate collaboration and cooperation within the medical devices network during the implementation phase of the new Regulations.
2 Development of medical device policies Figures and tables Figure 1. National health policy framework 10 Box 1. Elements of effective national health policies, strategies, and plans 11 Box 2. Medical devices that address Millennium Development Goals 4, 5, and 6 14 Figure 2. Number of deaths attributable to select noncommunicable and communicable diseases, 2004–2030 15 Figure 3. …
new Community-wide Regulations, one on medical devices and one on in vitro diagnostic medical devices. The texts of these new Regulations were adopted by the European Parliament in April 2014 and they are likely to come into force between 2018 and 2020. Unlike Directives, Regulations become binding automatically without the need to be implemented by national laws, so there is less scope for
The QS regulation applies to finished device manufacturers who intend to commercially distribute medical devices. A finished device is defined in 21 CFR 820.3(l) as any device or accessory to any
Medical Product Software Development and FDA Regulations Software Development Practices and FDA Compliance Medical Product Software Development and FDA Regulations Introduction Regulated Software FDA Overview Medical Device Definition Software – Special Attention Regulation Of Software Basic Requirements Software Quality Model Software Safety Model Software …
Faunce TA, Johnston K, Bambrick H. The The Trans-Tasman Therapeutic Products Authority: Potential AUSFTA Impacts on Safety and Cost-Effectiveness Regulation for Medicines and Medical Devices …
Health Law, Ethics, and Human Rights from The New England Journal of Medicine — Regulation of Medical Devices in the United States and European Union
US FDA Medical Device Premarket Procedures Presenter: Timothy A. Ulatowski VP, NSF Health Sciences 1 . ITA-FDA Medical Devices Regulatory Capacity Building Training Program for International Medical Devices Regulators March 27 – 28, 2014; San Francisco, California Overview Introductory Remarks Background on FDA medical device premarket organization A few basics of FDA medical device laws
IP considerations for medical devices IPR-Helpdesk
REACH AND ITS IMPACT ON MEDICAL DEVICES

Medical Devices US Law Regulation and Practice
Jamaica who.int

EU Medical Device and IVD Regulations Overview Series Part 1
Regulation of Medical Devices in the United States and
.jpg)
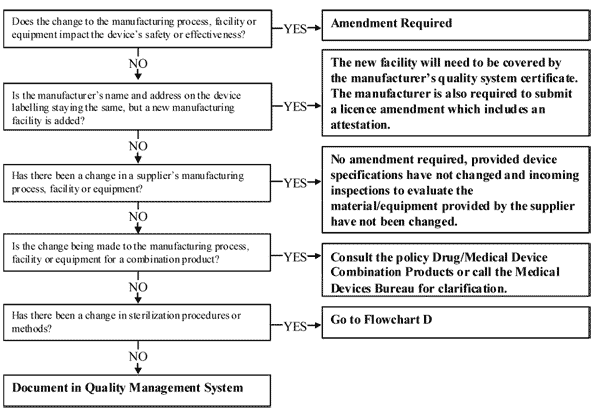
Published Medical Devices Regulation/In-vitro Diagnostics
The Trans-Tasman Therapeutic Products Authority Potential
– Medical Devices Regulatory Affairs Postgraduate
Public Accountability and Medical Device Regulation

Presentation New Direction of Japanese Regulations on MD
MEDICAL DEVICE INNOVATION IN AMERICA ENSIONS BETWEEN
Medical Devices Regulation Growth
Medical Devices Law & Industry Report Pinsent Masons
on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Council Directive 90/385/EEC of 20 June 1990 on the approximation of the laws of the Member States relating to active implantable medical devices (OJ L 189, 20.7.1990, p. 17).
regulation and law, affecting the development and ultimate marketing of new medical products, drugs, devices and biologics. They have served the regulatory needs of large (> billion/year)
US FDA Medical Device Premarket Procedures Presenter: Timothy A. Ulatowski VP, NSF Health Sciences 1 . ITA-FDA Medical Devices Regulatory Capacity Building Training Program for International Medical Devices Regulators March 27 – 28, 2014; San Francisco, California Overview Introductory Remarks Background on FDA medical device premarket organization A few basics of FDA medical device laws
Faunce TA, Johnston K, Bambrick H. The The Trans-Tasman Therapeutic Products Authority: Potential AUSFTA Impacts on Safety and Cost-Effectiveness Regulation for Medicines and Medical Devices …
“medical device family” means a group of medical devices that are made by the same manufacturer, that differ only in shape, colour, flavour or size, that have the same design and manufacturing process and that have the same intended use.
Regulations for the D evelopment of M edical D evice S oftware
Medical Devices Law & Industry Report Pinsent Masons
Regulations for medical device development Accelerate
The life-cycle of a medical device is calculated from its design and development to manufacture and its subsequent disposal. It can be divided into three common stages, namely pre-market, placement on-market and post-market. Safety and performance of a medical device may affect at each of these stages. It is therefore important that the scope of the regulatory control covers the entire life
Medical Devices Regulations WIPO
In November 2014, Japan introduced the Pharmaceutical and Medical Device Act (PMD Act), which made significant changes to the country’s medical device registration process.
Medical Devices Internal Market Industry
Regulation of Medical Devices in the United States and
Public Accountability and Medical Device Regulation
This Article analyzes the objectives and structure of the 1976 medical device law and concludes that one of the statute’s core values is the prin- ciple of public accountability for Agency actions (Part II).
Where is the new medical devices legislation?
David W Feigal, M.D., M.P.H. Senior Vice President for Global Regulation, Pharmacovigilance and Risk Management Élan Pharmaceuticals Regulations for medical
Medical Devices Regulation Growth
Faunce TA, Johnston K, Bambrick H. The The Trans-Tasman Therapeutic Products Authority: Potential AUSFTA Impacts on Safety and Cost-Effectiveness Regulation for Medicines and Medical Devices …
Public Accountability and Medical Device Regulation
Pharmaceutical and Medical Device Law Regulation of
Regulation of medical devices in europe – the role of