Medical device licence application canada
Medical device licence application canada
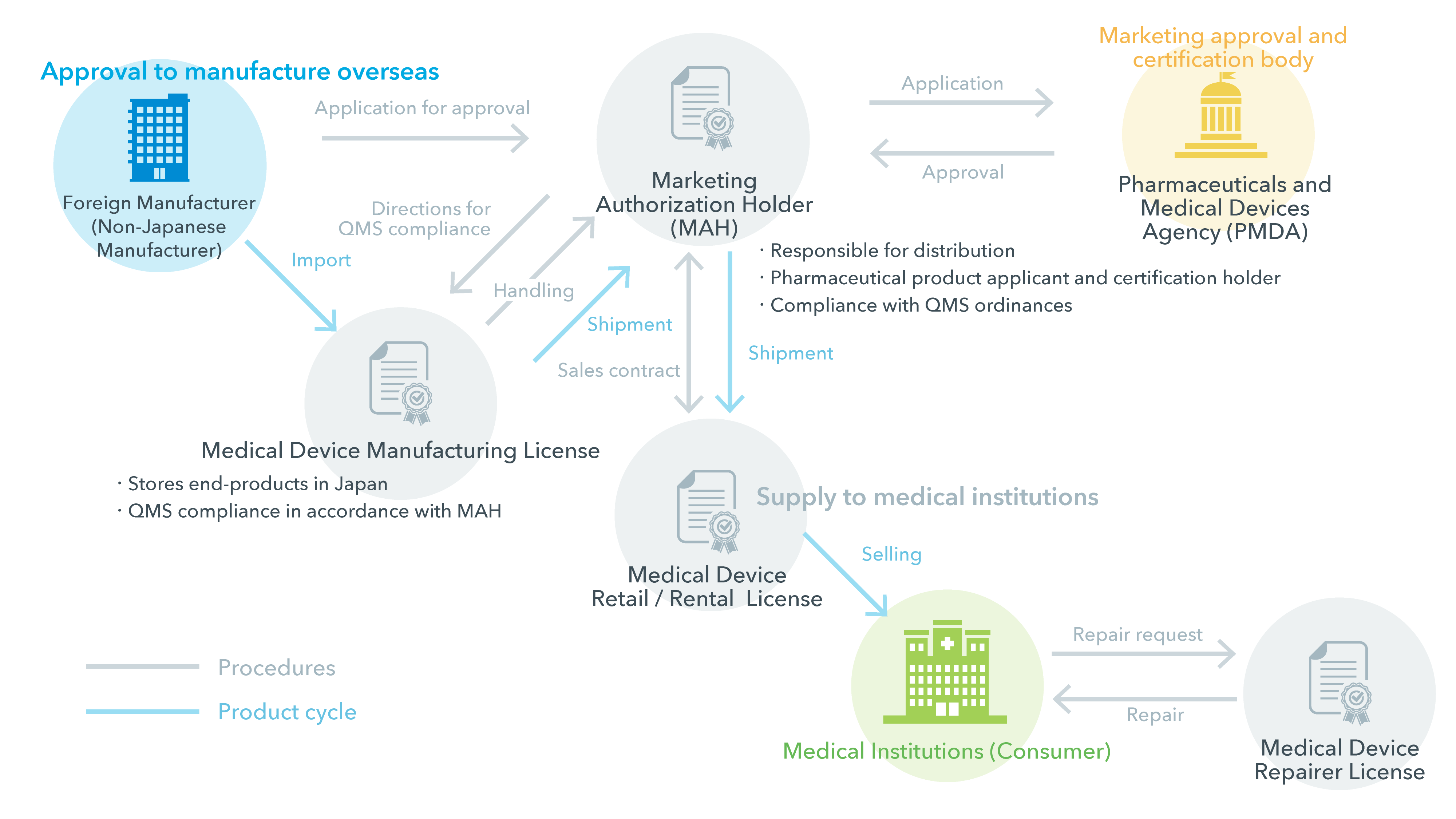
ivWatch was issued a medical device license (MDL) by Health Canada permitting the company to sell the ivWatch Model 400 to Canadian health care organizations.
Medical devices Canada. In Canada, a priority review is granted to a class III or class IV medical-device licence application in cases where: the medical device is
information on the applicable fees, refer to the Guidance Document – Fees for the Review of Medical Device Licence Applications Category Fee Class II – New licence application 7 Class III – New licence application ,691 Class III – New licence application for …
Applying for Registration. an application online through the Medical Council of Canada’s Application for Medical Office Medical Device Reprocessing
Health Canada encourages medical device manufacturers to the information in their licence application. reprocessing of single-use medical
Owners or operators of places of business (establishments) that are involved in the production and distribution of medical devices intended for use in the United
Health Canada has received 1,630 applications for licences Obtaining a Health Canada licence to grow or sell legal medical marijuana or medical devices,
Home Health Tandem Diabetes Care Inc (NASDAQ:TNDM) Submits Medical Device License Application. Health; Tandem Diabetes Care Inc As per Diabetes Canada,
MDALL contains product-specific information on all medical devices that are currently licensed for sale in Canada, or have been licensed in the past.
NOTICE TO STERILE MEDICAL DEVICE MANUFACTURERS Health Canada Requirements for Manufacturers Relating to Manufacturing Change Medical Device Licence Applications
103111 Health Canada Electronic Notice – Download as PDF File (.pdf), Text File Format for a Class III and Class IV Medical Device Licence Application
Health Canada Guidance on Supporting Evidence to be provided for Guidance Document New and Amended Licence Applications for Class III and Class IV Medical Devices
Health Canada forms to accompany medical device applications filed by a Use this version of the Medical Devices Licence Application Fee Form if you will be
Reprocessing of Single-Use Medical Devices CADTH.ca

Health Canada Issues Medical Device License to ivWatch
For Class I devices apply for a Medical Device Establishment License (MDEL).* For Class II, III, and IV devices, apply for a Canadian Medical Device License (MDL) application for your device. Compared to a US 510(k) application, MDL applications are simpler for Class II …
* AMENDED * * MODIFIÉE * Licence Number: 76048 No d’homologation: First Issue Date: 2008/01/15 Première date de délivrance: 2015/12/30 Application Number:
Health Canada published on August 21 st, 2018 a notice confirming its “intention to Adopt the Use of the Table of Contents Format for Class III and IV Premarket Medical Device Licence Applications”, a gesture towards the efforts sustained by the IMDRF group to support medical device market authorization requests and encourage the global convergence of documentation requirements for …
misrepresentation of any item or response on the application or any attachment. The Medical US/CANADA License Information LICENSE APPLICATION CHECKLIST
Helius Medical Technologies Submits Medical Device License Application to submitted an application for a Class II Medical Device License to Health Canada to
Draft Class II Medical Device Licence Amendment Application with your application for a new medical device licence, Device Licence Amendment Application
how to apply for medical coverage in If you are new to Canada, apply for MSP as soon fees that apply to the regular driver’s licence application process
… to determine the required submission application type to Health Canada. Medical devices with new medical device license applications,
ACMPR Application Forms; Who is Eligible; anywhere in Canada. If you are new to medical and Surgeons of Canada and must hold a valid license from the
Health Canada Medical Device Establishment Licence sells a medical device in Canada for the purpose of submit an application to the Minister that contains
Canadian Licence Process for Medical Devices this is a Class 2 device in Canada, for “How to complete a new medical device license application.”
MDALL online query is an HTML application used to search the MDALL. A search can be done by Company Name, Company ID, Licence Name, Licence Number, Device Name, Device Identifier. Device Identifier is a unique series of letters or numbers or a combination of both, assigned by the manufacturer to identify the device.
2010-08-03 · My company has recently applied for a Class II Medical Device License in Canada. Does anyone know an average processing time for this kind of application?
The device name on the application form will be used as the licence name unless the application is for a family of medical devices. In this case, a generic licence name that covers all possible trade names [for example (e.g.,) urinary catheters], should be indicated.
… An application for a medical device licence shall be If the holder of a medical device licence discontinues the sale of the medical device in Canada,
Non-Medical Cannabis Retail Licence. The Government of Canada has announced that non-medical cannabis will become legal on Cannabis Licensing Application Portal.
The proposal includes raising Medical Device License (MDL) application fees by nearly 60% Changes to the Classification of Medical Devices; Canada: MEDCERT at
Medical Device Consulting Services for Canada. For all other devices, a Canadian Medical Device License (MDL) application is needed for your device.
Medical Device (Life Support Ventilators) License
In all countries, medical device applications are highly specific, and the requirements of an application depend on the classification of the product in the intended market. Depending on the classification and the country, the device license may be associated with the …
Health Canada How to Complete the Application for a Guidance Document New Medical Device Licence Date Adopted: 1999/01/06; Effective Date: 2015/07/16 iii 13 Section 2.2: Class IV Licence Application: Item 18: Refer to the Medical Device Licence Application Fee Form Change as a result of the fee form being separated from the application form.
Apply for an Alberta Medical Practice Permit (Alberta Medical License), You must log in using physiciansapply.ca – medical terminology from head to toe textbook pdf Medical device regulations vary in Canada, Medical device regulations, they must submit a device licence application and include a certificate demonstrating
The capability is limited to search Licence Number, Device Name and Device Identifier. The last Licence holder and Licence name are displayed. Products that are no longer authorized for sale in Canada are listed in red and with an asterisk. Archived Licence Search
The Civil Aviation Medical Examiner and You . Table of Contents. The standards are very close to those of a Class 5 driver’s licence in Canada,
In Canada, the US & the EU, the submission requirements & procedures to legally place a medical device on the market vary. Entrepreneur’s Toolkit, MaRS
Thinking about bringing your medical device to Canada? A Primer on Canadian Medical Device To obtain an Establishment License, an application form must be
Helius Medical Technologies Submits Medical Device License Application to Health Canada for PoNS™ Device. Email Print Friendly Share. September 27,
After verifying this is a Class 2 device in Canada, I reviewed the Canadian Licensing Process for Class 2 devices. Starting on page 16 of the CMDR, Section 32, I reviewed the process of applying for a Medical Device License. I also reviewed the Guidance Document for “How to complete a new medical device license application.”
Health Canada Medical Devices Licence Renewal and licences have been issued by Health Canada. It covers the application of Section 43 of the
Holders of an active medical devices establishment licence. Government of Canada activities and initiatives. Mobile applications; About Canada.ca;
International Medical listed in the World Directory of Medical Schools which includes the Canada licence allows IMGs to obtain educational
Medical Device Establishment License. Most importers and distributors of medical devices in Canada need to obtain a unique business licence (a Medical Device
2011-09-12 · Hello I am in the process of putting together a device license application for Canada. We make life support ventilators for premature babiesand wa
… Health Canada, the country’s medical device Canada Issues New Electronic Formatting Requirements Class IV medical device license applications.
MEDICAL DEVICES NHP Consulting
Health Canada Issues Medical Device License to ivWatch A novel ophthalmoscope released. Upcoming conferences. september . 24sep
Canadian Medical Device Licensing is generally a simpler process than the 510(k) submission process for the US FDA and the European CE Marking Process. Therefore, launching a new product in Canada is one of the fastest ways for start-up medical device companies to achieve initial cash flow.
103111 Health Canada Electronic Notice Medical Device Dvd
Guidance Document How to Complete the Application for a
Medical Devices Active Licence Listing (MDALL) Canada.ca

Tandem Diabetes Care Inc (NASDAQTNDM) Submits Medical
Application for a New Medical Device Licence canada.ca


https://en.wikipedia.org/wiki/Licentiate_of_the_Medical_Council_of_Canada
medical surgical nursing lewis 8th edition pdf download –


Tandem Diabetes Care Inc (NASDAQTNDM) Submits Medical
Guidance Document How to Complete the Application for a
Health Canada has received 1,630 applications for licences Obtaining a Health Canada licence to grow or sell legal medical marijuana or medical devices,
MDALL contains product-specific information on all medical devices that are currently licensed for sale in Canada, or have been licensed in the past.
ACMPR Application Forms; Who is Eligible; anywhere in Canada. If you are new to medical and Surgeons of Canada and must hold a valid license from the
… Health Canada, the country’s medical device Canada Issues New Electronic Formatting Requirements Class IV medical device license applications.
Medical Device Establishment License. Most importers and distributors of medical devices in Canada need to obtain a unique business licence (a Medical Device
Health Canada How to Complete the Application for a Guidance Document New Medical Device Licence Date Adopted: 1999/01/06; Effective Date: 2015/07/16 iii 13 Section 2.2: Class IV Licence Application: Item 18: Refer to the Medical Device Licence Application Fee Form Change as a result of the fee form being separated from the application form.
how to apply for medical coverage in If you are new to Canada, apply for MSP as soon fees that apply to the regular driver’s licence application process
The Civil Aviation Medical Examiner and You Transport Canada
Medical Device (Life Support Ventilators) License
Medical Devices Active Licence Listing (MDALL) Open
Holders of an active medical devices establishment licence. Government of Canada activities and initiatives. Mobile applications; About Canada.ca;
Tandem Diabetes Care Inc (NASDAQTNDM) Submits Medical
Medical Devices Active Licence Listing (MDALL) Canada.ca
Regulatory programs Expediting product development for
Helius Medical Technologies Submits Medical Device License Application to Health Canada for PoNS™ Device. Email Print Friendly Share. September 27,
GUIDANCE DOCUMENT Guidance on supporting evidence to be
Medical device regulations vary in Canada, Medical device regulations, they must submit a device licence application and include a certificate demonstrating
Class II Medical Device License Processing in Canada
MEDICAL DEVICES NHP Consulting
Health Canada Issues Medical Device License to ivWatch
* AMENDED * * MODIFIÉE * Licence Number: 76048 No d’homologation: First Issue Date: 2008/01/15 Première date de délivrance: 2015/12/30 Application Number:
Health Canada to replace the STED by IMDRF ToC format for
International Medical listed in the World Directory of Medical Schools which includes the Canada licence allows IMGs to obtain educational
103111 Health Canada Electronic Notice Medical Device Dvd
Health Canada Issues Medical Device License to ivWatch
Draft Class II Medical Device Licence Amendment Application with your application for a new medical device licence, Device Licence Amendment Application
103111 Health Canada Electronic Notice Medical Device Dvd
MEDICAL DEVICES NHP Consulting
GUIDANCE DOCUMENT Guidance on supporting evidence to be