Medical device marketing strategy pdf
Medical device marketing strategy pdf
Medical Device Post-Marketing Studies in Europe Vytis Jusevicius Projects Director AptivSolutions 26th Annual EuroMeeting 25-27 March 2014 ACV, Vienna
As we mentioned in our previous blog, Medical Device Product Launch: 4 Critical Planning Steps, the top four challenges faced by a new medical device product launch team include planning, communications, implementation, and follow-up.
Medquip, Inc. medical equipment developer business plan executive summary. Medquip, Inc. is a start-up business that will develop and market endoscopic medical devices through multiple distribution channels, both foreign and domestic.
THE MEDICAL DEVICE MARKET LOOKS PROMISING DUE TO AN AGING AND MORE DEMANDING POPULATION BUSINESS SWEDEN 2 A NEW WAY OF LIFE China’s aggressive healthcare reform plan from 2009 to bring all its citizens under basic medical coverage
This is a marketing strategy for a leading medical device company for a new product launch. This presentation won the Babson Marketing Case Competition – 2012 with prize money of 00. 16 schools across the globe were competing in this competition.
Whether engaged in developing and marketing new medical devices or repurposing and re-marketing existing ones, a number of critical hurdles must be overcome. Before the ideation and creation of a novel prototype device — or the establishment of
Clinical Strategy Review The clinical evaluation is a critical element in the CE marking regulatory pathway for placing a new medical device on the European market.
Medical Device Challenges By Robert G. Launsby Launsby Consulting April, 2011 A number of innovative new medical products have been recently developed that have a dramatic impact on our quality of life. One example is a nebulizer that utilizes vibrating mesh technology. A nebulizer is a medical product that has been used as a way of transforming respiratory medication from liquid form …
Strategy Inc. provides comprehensive medical device consulting services, such as customizable market analyses and financial due diligence. We have expertise in voice of the customer evaluations, market assessments, valuations, competitive analyses, and more.
Optimising your Regulatory Strategy to gain FDA and EU Approval Roger G. Harrison, PhD. Drug-Device and Biologic Combination Products, 2005
regulatory strategy is to ensure an efficient of alaunch medical device in the countries in scope by reducing time to submission and contribute to a rapid and cost-efficient market access.
Medical device and diagnostic companies develop some of the most technologically advanced products, but often their marketing tactics lag behind other high-tech industries. Despite the limitations of marketing in a highly regulated environment, there’s an opportunity for medical device and diagnostic companies to stand out in their respective spaces with a solid digital marketing strategy.
The creation of a marketing plan for medical devices products for Myanmar market has to be based on a detailed analysis of the product itself and the produced company, i.e. the quality
A Qualitative Study: Clinical Marketing Strategies for Medical Devices Innovations that Increase Adoption in the USA By Victoria J. Smith BSc., Behavioural Neuroscience, 2010
OPPORTUNITIES IN THE MEDICAL DEVICE MARKET
Digital Marketing Strategies for Medical Device and
According to the Medical Device Act, a medical device is defined as an instrument, machine, device, material, or any other similar product specified below as one …
medical device space; for example, hospital utilization of medical devices has slowed significantly, reimbursements continue to tighten, and there was a first-time global decrease (5%) in US premarket approvals (PMAs) in 2012, a trend that continued into
To medical device manufacturers and the vendors that serve them, Joe Hage is the medical device marketing consultant that will increase the quality and quantity of your medical leads because, before he started his practice in 2011, he helped a 0-million medical device manufacturer create an entirely new web presence and strategy, increase page views by 253 percent, introduce social media
Attend Our Seminar to Discover the Secrets to Attract Patients and Build Your Brand. When you attend our Advanced Healthcare Marketing Strategies seminar, you will discover secrets to successful digital marketing, doctor referral building, patient referrals, branding, free press, advertising and more.
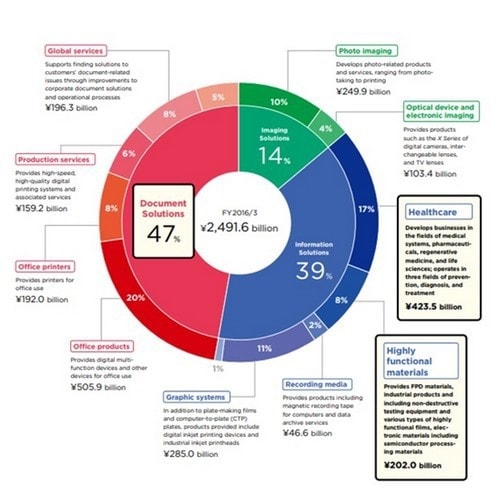
Today’s Agenda 3 2016/3/30 1. Review of Medium-Term Vision (From FY2013) for Medical Business 2.Recognition of Current Conditions 3.Directives for Medical Business Strategy
shifting their innovation strategies from incremental toward breakthrough innovation. Pouring more dollars into research and development (R&D) alone, however, is not enough. As the survey results on the following pages show, medical device companies need to review their traditional approaches to innovation and take up new strategies designed to accommodate the rapidly evolving and globalized
The FDA now takes as much as 140 days to process medical device marketing submissions, according to USDM Life Services Make sure you plan this time into your selling strategy to prevent buffers in the flow of sales.
Offline marketing, or traditional marketing, should always be included in the medical office marketing plan. Although many patients are using the internet to find a new practice or reconnect with an old one, traditional marketing is still an effective approach to reaching out to patients.
The Key Challenges FaCing MediCal deviCe ManuFaCTurers (and how to meet them with strategic interoperability) Executive Overview accountable care and value-based reimbursement models are causing a seismic
In this article, we will examine the variances between device and drug marketing, and how specific differences translate into opportunities, strategies, and approaches. Four key differences By understanding the key differences between device and drug promotion, marketers can maximize their effectiveness with customers.

Indian Medical Device Industry – Current State & Opportunities for Growth – Dinesh Peter, Life Sciences and Services, Infosys Consulting . External Document 2017 Infosys Limited External Document 2017 Infosys Limited Introduction The Indian medical device market is worth well over the billion dollar mark and is on a path of lower double digit growth as of 2014. Despite the presence of a
When formulating the device PMS plan, it is pertinent to remember that ISO 13485 applies to all medical devices on the market and in the context of this …
Device manufacturers experiencing declining growth or narrowing margins may need to reevaluate their commercial models. The rules of the road are changing for the medical-device industry. While strong demand, demographics, and value still generate interest and investment, shifting expectations are
A ready “Sales and Marketing department” Plan, propose, and execute demand generation activities Distributor management, drive commitment to our brand Possible advocacy to shape policy, create long term, multi stakeholder PPPs – positioning company as “knowledge partner”, to address large social health issues, while embedding select technologies as an integral part of the solution
called a “Marketing Authorization Holder (MAH).” An MAH must be physically located in Japan. The MAH must obtain marketing approval (hanbai shonin) for each product. A U.S. manufacturer intending to manufacture medical devices in the United States and export them to Japan is required to be registered by the Pharmaceutical and Medical Device Agency (PMDA) as a “Registered Foreign
medical device development and commercialization. The team of speakers that constructed this program The team of speakers that constructed this program holds an impressive history of broad based expertise and successful implementation in medical device
such as business background, marketing plan, financial plan, operational plan, etc. The conclusion provides useful and valuable information to the reader about the process of establishing the new medical centre in Eastern Finland.
4 BMI South Africa Medical Devices Report Q1 2014 Research to guide the development of strategy for the medical devices sector of South Africa Page 9 of 75 devices Sector.
A medical device is defined by law in the section 201(h) of the FD&C Act, and the classification, which may be found in the Code of Federal Regulations, determines the regulatory path and
USCAN Marketing Manager “The Emerge Inside Sales Program was the single best marketing investment we have made.” Paul Walker, MBA Marketing Manager, New Product Development “I used Mike and his Emerge team for a new business development initiative at BD Medical to supplement our national sales force in their efforts to make a big splash into a new target segment. His team …
its next five year strategic economic plan (2016–20), Overall Rank 28 . Malaysia represents one of the more vigorous and vibrant medical device markets in Southeast Asia, presenting opportunities for U.S. exporters of medical technology to expand their sales into rising economies. Increasing patient access to healthcare will remain in the focus of the Government of Malaysia for the next five
The growing demand for products that are “good enough” and competitively priced has pushed medical-product manufacturers to develop strategies to attract and retain this new segment of customers. The medical-device industry is facing challenging headwinds. As governments and health insurers
Strategic Market Creation Plan (Roadmap) Fulfill the control tower function in R&D in government sectors Drastically improve international competitiveness and power of R&D in private sectors Establish systems and rules to secure safety which adopt to the next generation …
STRATEGIC SALES AND MARKET PLANNING: IN TODAY’S ECONOMY Focus on Medical Devices 2. THE MEDICAL DEVICE INDUSTRY• Definition: The United States medical device manufacturing sector is a highly diversified industry that produces a range of products designed to diagnose and treat patients in healthcare systems worldwide.
Medical Device Marketing – Best Practices, Strategies, and Tips Webinar Transcription [Slide 1] Okay, this is Joe Hage. I am the leader of the Medical Devices
Our team has launched over 500 healthcare brands and received dozens of awards for medical marketing strategy and advertising.
a patent strategy that complements the FDA rules and provides the best market protection for medical products. fDA Basics The FDA provides significant barriers to market entry because drugs, biologics, and certain medical devices cannot be placed on the market without prior FDA approval, and they must be proven to be safe and effective before approval will be given. Furthermore, before a
New Commercial Models in Medical Devices Healthcare reform, new sales models, emerging social channels and . draconian cost-cutting imperatives are forcing medical device companies to transform how they operate. By embracing a holistic model, these companies can remake their commercial operations. Executive Summary. Enormous change is on the horizon for medical . device (MD) …
Medical Device Challenges Launsby Consulting
Sample Medical Device 30/60/90 Day Plan OBJECTIVE OF THE POSITION: Bring the Iowa and Nebraska territory of the “Medical Products Company” Urology Division to …
THE WHITAKER INSTITUTE ! MEDICAL DEVICE SECTORAL OVERVIEW 6 ! Executive Summary Overview of the Medical Device Sector Global Overview • The current global market is valued at 8 billion, up from 4 in 2010 and
Developing an innovative healthcare product (a drug or a biologic, or a medical device) from the proof-of-concept stage to the marketing stage is an expensive and complex process. It involves
strategy, advice and support services to medical device, diagnostic and clinical research clients. He was previously Manager, Reimbursement & Outcomes Planning at St. Jude Medical, Corporate Reimbursement and Health Policy. Prior to that, he was Principal Reimbursement Advisor at Boston Scientific, Health Economics and Reimbursement. He also holds a Master’s degree in Management … – medical dictionary translator english spanish Business Presentation for Medical Systems Business November 26, 2013 FORWARD-LOOKING STATEMENTS Forward-looking statements, such as those relating to earnings forecasts and other projections contained in this material, are
MedNexis, Inc. medical equipment business plan strategy and implementation summary. MedNexis, Inc. is a start-up medical device company that has designed and patented devices to aid in atrophy treatment/prevention.
Medquip, Inc. is a medical device development company that intends to design,. Successful Successful implementation of sales and marketing plan to U.S. managed care .
knowledge, and tools at all stages of a device’s development, evaluation, and marketing. The Medical Device Safety Action Plan: Protecting Patients, Promoting Public Health outlines a vision for
The industry of medical device sales is known to be very tough to succeed in; however individuals who do can reap great rewards. I am interested in pursuing a career in medical device sales and want to get a broad overview of the industry and its norms. I’ve decided to answer this question
September 29, 2014 Articles, Marketing Strategy, Medical Device Marketing 0 On the morning of September 16th, DevicePharm welcomed dozens of Orange County early-stage medical technology executives as the host of an OCTANe program titled, “How to Successfully Launch a Medical Device …
“How to Successfully Launch a Medical Device Brand” – Key
ENTREPRENEUR GUIDES MaRS Discovery District

Optimising your Regulatory Strategy to gain FDA and EU
MEDICAL DEVICE REIMBURSEMENT

Medical Equipment Developer Business Plan Sample
The Medical Device Industry in Korea Strategies for


How to Optimize Your Medical Device Sales Process [Free
Research to guide the development of strategy for the
– Medical Device Post- Marketing Studies in Europe
MARKETING STRATEGIES FOR MEDICAL DEVICES MARKET IN

Medical Device Commercialization Getting Great Ideas to
Provisional Strategic Market Creation Plan (Roadmap)
Marketing Strategy for Medical Devices Market CORE
Regulatory strategy for an efficient launch of medical
STRATEGIC SALES AND MARKET PLANNING: IN TODAY’S ECONOMY Focus on Medical Devices 2. THE MEDICAL DEVICE INDUSTRY• Definition: The United States medical device manufacturing sector is a highly diversified industry that produces a range of products designed to diagnose and treat patients in healthcare systems worldwide.
Business Presentation for Medical Systems Business November 26, 2013 FORWARD-LOOKING STATEMENTS Forward-looking statements, such as those relating to earnings forecasts and other projections contained in this material, are
A Qualitative Study: Clinical Marketing Strategies for Medical Devices Innovations that Increase Adoption in the USA By Victoria J. Smith BSc., Behavioural Neuroscience, 2010
knowledge, and tools at all stages of a device’s development, evaluation, and marketing. The Medical Device Safety Action Plan: Protecting Patients, Promoting Public Health outlines a vision for
4 BMI South Africa Medical Devices Report Q1 2014 Research to guide the development of strategy for the medical devices sector of South Africa Page 9 of 75 devices Sector.
To medical device manufacturers and the vendors that serve them, Joe Hage is the medical device marketing consultant that will increase the quality and quantity of your medical leads because, before he started his practice in 2011, he helped a 0-million medical device manufacturer create an entirely new web presence and strategy, increase page views by 253 percent, introduce social media
STRATEGIC SALES AND MARKET PLANNING: IN TODAY’S ECONOMY Focus on Medical Devices 2. THE MEDICAL DEVICE INDUSTRY• Definition: The United States medical device manufacturing sector is a highly diversified industry that produces a range of products designed to diagnose and treat patients in healthcare systems worldwide.
Strategic Sales And Market Planning SlideShare
Developing an innovative healthcare product (a drug or a biologic, or a medical device) from the proof-of-concept stage to the marketing stage is an expensive and complex process. It involves
Medical Device Marketing Plan Strategy Questions
its next five year strategic economic plan (2016–20), Overall Rank 28 . Malaysia represents one of the more vigorous and vibrant medical device markets in Southeast Asia, presenting opportunities for U.S. exporters of medical technology to expand their sales into rising economies. Increasing patient access to healthcare will remain in the focus of the Government of Malaysia for the next five
Marketing Strategy for a medical device company SlideShare
As we mentioned in our previous blog, Medical Device Product Launch: 4 Critical Planning Steps, the top four challenges faced by a new medical device product launch team include planning, communications, implementation, and follow-up.
A Qualitative Study Clinical Marketing Strategies for
Medical Device Marketing Plan Strategy Questions