Medical device quality plan example
Medical device quality plan example
Medical Device Quality We will focus on the PDP quality plan in this example and the remediation David Amor is a medical device consultant who has
Implementing and maintaining a quality management system For small medical device manufacturers in the pre Do we need a QMS if we plan to outsource
Quality Management System (QMS) Plan is an important process and Quality Teams in Medical Device of implementing a new quality management system (QMS)
ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related
QUALITY PLANS. A quality plan is a document, or several documents, An example of this is a manufacturing company that machines metal parts.
What it is: Develop Quality Management Plan . Description The Quality Management Templates/Examples: Develop Quality Management Plan. Introduction
production of custom control panels and input devices. Excluded clauses from ISO 13485 4.1 The company’s executive management reviews the quality system and plan
Learn how Arbour Group established a corporate computer system quality policy and related procedures to ensure compliance across an entire corporation.
Best practices for successful product launch implementation and follow up for medical device 7 Steps to Successful Medical Device Product Launch Plan : 1. Set
Holding a management review meeting is one of the most establish a quality plan which defines reviews as a manufacturer of a medical device.
ISO 13485 quality system plan Medical Device Academy

ISO 134852016 Medical devices — Quality management
article explains how to write a quality system plan template in order to revise and update your quality system for compliance with ISO 13485:2016
… for the company’s Strategic Quality Plan and other company quality within the Strategic Quality Plan. Product and/or Process Quality Planning.
Medical Device Quality Agreement Template Prepared by regulatory framework applied to the medical device. Send the Base Control Plan to potential
Quality System: Design Control Procedure “Medical Device Quality Systems Manual, Quality System: Design Control Procedure – Appendix Page 6 of 10
21/08/2016 · Can anyone provide a template for Design and Development Plan for a medical device?

Home › Online Training › Medical Devices › Supplier Quality Agreements for Medical Devices Quality-Control-Plan. Template for a Software Maintenance
The primary intention of the Quality Plan was to demonstrate how the QMS (Quality Management System) is applied to a specific case, for example, when the company is
Quality Risk Management – The Medical Device Experience Medical Devices Quality Risk Management Plan Information Collection
What do quality system requirements cover? Medical device manufacturers who plan to take their product to the global marketplace should carefully review the ISO 13485
Medical Device Academy, Inc.Quality System Plan Template. Page 1 of 3Rev A – November 19, 2015
A Standard Operating Procedure (SOP) template as word document Standard Operating Procedure Templates – SOPs. most important SOPs of a Medical Device Quality
Construction Quality Control Plan Templates From simple to comprehensive. Download a Quality Plan Sample and see what you’ll get in each section of your QC Plan.
ISO 13485:2003 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related

Corrective and Preventive Action – Inspection of Medical Device Manufacturers • Premarket Approval • Develop action plan for corrective action and
Medical device QMS/GMP system and audit medical device manufacturer’s quality management system and 4.1 Plan for Measurement,
Integrated Quality and Risk Management Plan It is recommended that the baseline RACT output and any revisions are maintained and documented. Example
MEDICAL DEVICE QUALITY requirements that all manufacturers and distributors must consider when they plan to manufacture medical devices, examples that firms
Systems of Medical Device Manufacturers – Part 2: conducting medical device quality Quality Systems of Medical Device Manufacturers Part 1
Quality Management System Certification The international working group has proposed a work plan for Once a medical device quality management system has been
Construction quality assurance/quality control (QA/QC) plan samples for commercial, industrial & civil construction. Download now!
ISO 13485 2016 Translated into Plain English praxiom.com
Downloadable QMS templates. Medical Device Quality Management System The sample on the website really helped sell the templates to me.
A Quality Plan is essentially a document plan that what Quality Planning do we need? Medical Device and Biotech industry and risk not being seriously
4 Important Medical Device Quality Assurance and Management Get your quality plan correct; Invest in an Integrated Medical Device Quality Assurance Software
The valid rational in developing statistical sampling for design verification and validation of a Quality Limit (AQL sample plan for medical device – medical dictionary english to gujarati free download pdf A medical device quality plan is intended to ensure that relevant quality standards are met when manufacturing a medical device, and that the medical
Quality Plan: This Quality Control Plan and Quality Assurance Plan template helps you to gain project quality management improvement within your business. Use this
Supplier Quality Agreements © 2009, for example, plating of metals, and consultants selected by manufacturers of medical devices
A FDA Quality Plan (QP) or Validation Plan (VP) If you are in the Pharmaceutical / Biotechnological / API / Medical Device,
This article is a real-time case study that explains how to implement a new ISO 13485 quality system plan at an accelerated schedule of just four months.
Start here. Preparing a QMS compliant with ISO 13485 or QSR 820 Thank you for purchasing the PharmOut Medical Device Quality Management System (QMS) template pack.
Learn About Quality / Quality Tools & Templates ; Quality Tools & Templates. Use this template to evaluate the potential failure of a product or process and its
Quality Control Plan Outline. Quality Control Plan Details (Example) QC Plan Template Last modified by: Taylor, Sharon D. Company:
Product development lifecycle: Medical device design Product development lifecycle: Medical device Establish and maintain a plan that describes the
ISO 13485 2016 is an international quality management standard for medical devices. This page presents an overview of ISO 13485 2016 and provides a PDF sample of
SOPs for Medical Devices; This Medical Devices Development Plan This Medical Device SOP Package contains the most important SOPs of a Medical Device Quality
ORA Inspectional References Guide to Inspections of Quality Systems. compliance with the Quality System Medical Device Quality Systems
The AMR Quality Improvement Plan For example, we constantly monitor our transportation providers to ensure that the vehicles and drivers serving our
17/04/2008 · The Medical Device Quality Plan. I got stuck with writing a quality plan for our products, My example quality plan:
Description. Why it is useful. A design and development plan is required to ensure that the design process is appropriately controlled and that device quality
Supplier Quality Agreements Ombu Enterprises
9/08/2013 · Does anyone have a quality plan template I can use for producing a new medical device? Thanks.
Guidance on the control of products and services obtained from suppliers. GHTF/SG3/N17R9:2008 December 11, 2008 Page 4 of 21 Introduction
Quality Procedures and Work Instructions Manual 9.4 Risk Assessment of Patient Care Devices Quality Procedures and Work Instructions, hereafter referred to as
These templates provide a professional framework to developing a Medical Device Quality Management system. What’s included in the Medical Device QMS template pack?
Design and Development Plan for a Medical Device
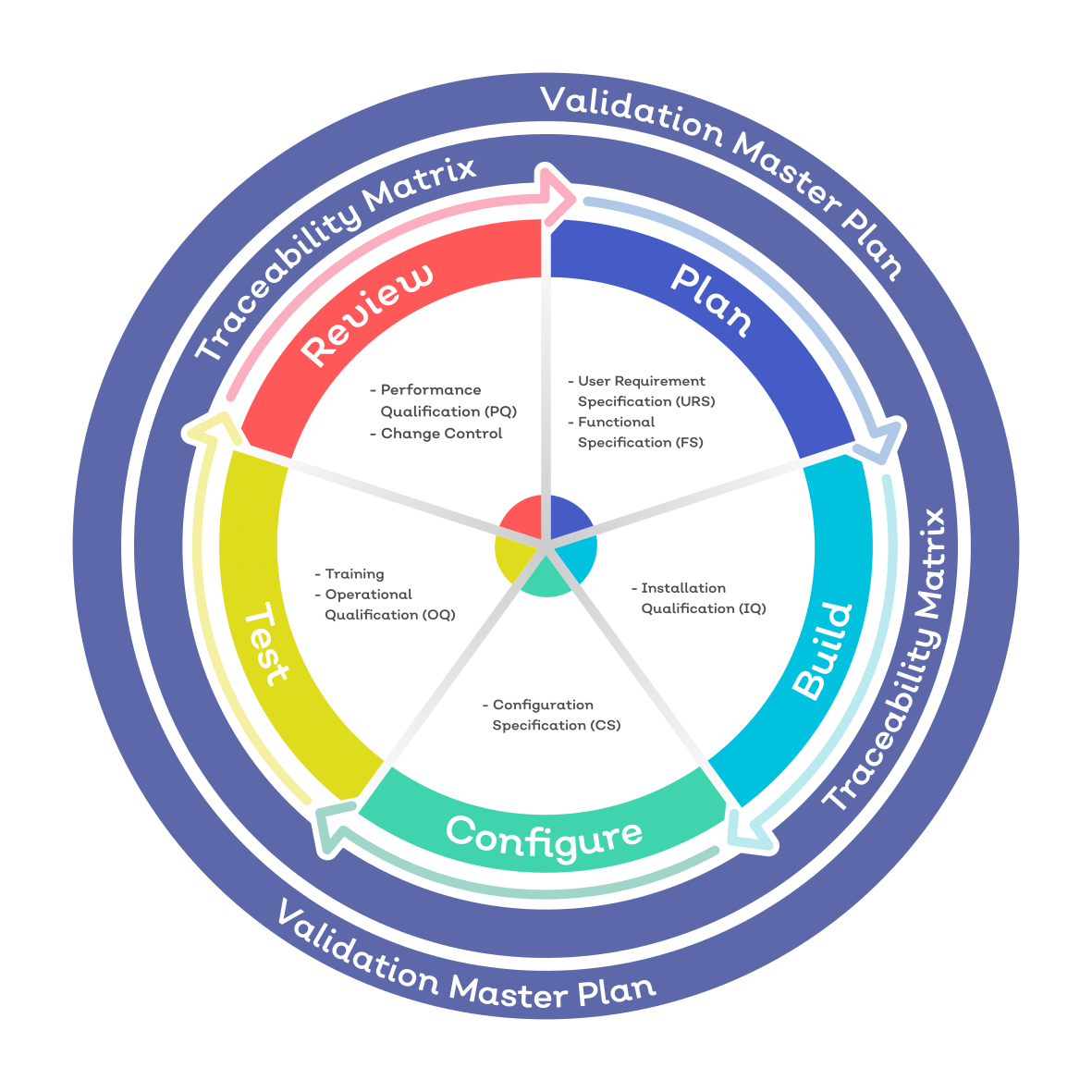
Quality system Medical device product development MaRS
An essential capability and competitive edge for manufacturers Medical device quality
Pharmaceutical Quality Management System Templates the template pack includes 40 The medical device QMS templates are used by our consultants in the field
Save time and money and make complying with regulations easy with our high quality professional plan template (medical device and for medical device
iso 13485 2016 translated into plain english 5. mar 2016 plain english quality management standard for medical devices edition 1 19 plan your quality policy.
Get expert help from a biotech or medical device business plan consultant. We can help with financial forecasts and pitch decks too! Established in 2001.
Quality Systems Food and Drug Administration

Project and Risk Management for medical devices Gantus AB
Quality Assurance/Quality Control Plan: Samples and Manual for Development Samples and Manual for Quality Assurance / Quality Control Plan 8 1. Check a sample of
Risk Management for Medical Devices (Quality, Design Control & Risk Free Risk Management Plan Template
GMP Documents and SOP Templates Medical Devices

SOPs for Medical Devices qm-docs.com
Quality Tools and Templates ASQ
medical radiation science curtin handbook – Download Sample Construction QA-QC Plan First Time Quality
Statistical Sampling Plan for Design Verification and


Quality Assurance/Quality Control Plan Samples and REC
www.medicaldevicesgroup.net
Design and Development Plan for a Medical Device
Statistical Sampling Plan for Design Verification and
QUALITY PLANS. A quality plan is a document, or several documents, An example of this is a manufacturing company that machines metal parts.
ISO 13485 2016 is an international quality management standard for medical devices. This page presents an overview of ISO 13485 2016 and provides a PDF sample of
A medical device quality plan is intended to ensure that relevant quality standards are met when manufacturing a medical device, and that the medical
Quality Control Plan Outline. Quality Control Plan Details (Example) QC Plan Template Last modified by: Taylor, Sharon D. Company:
Guidance on the control of products and services obtained from suppliers. GHTF/SG3/N17R9:2008 December 11, 2008 Page 4 of 21 Introduction
What it is: Develop Quality Management Plan . Description The Quality Management Templates/Examples: Develop Quality Management Plan. Introduction
Quality Risk Management – The Medical Device Experience Medical Devices Quality Risk Management Plan Information Collection
An essential capability and competitive edge for manufacturers Medical device quality
Best practices for successful product launch implementation and follow up for medical device 7 Steps to Successful Medical Device Product Launch Plan : 1. Set
Downloadable QMS templates. Medical Device Quality Management System The sample on the website really helped sell the templates to me.
Construction Quality Control Plan Templates From simple to comprehensive. Download a Quality Plan Sample and see what you’ll get in each section of your QC Plan.
These templates provide a professional framework to developing a Medical Device Quality Management system. What’s included in the Medical Device QMS template pack?
… for the company’s Strategic Quality Plan and other company quality within the Strategic Quality Plan. Product and/or Process Quality Planning.
Systems of Medical Device Manufacturers – Part 2: conducting medical device quality Quality Systems of Medical Device Manufacturers Part 1
Get expert help from a biotech or medical device business plan consultant. We can help with financial forecasts and pitch decks too! Established in 2001.
MEDICAL DEVICE QUALITY requirements that all manufacturers and distributors must consider when they plan to manufacture medical devices, examples that firms
ISO 13485 quality system plan Medical Device Academy
Supplier Quality Agreements Ombu Enterprises
ISO 13485 2016 is an international quality management standard for medical devices. This page presents an overview of ISO 13485 2016 and provides a PDF sample of
Quality system Medical device product development MaRS
… for the company’s Strategic Quality Plan and other company quality within the Strategic Quality Plan. Product and/or Process Quality Planning.
Medical Device Blog The Medical Device Quality Plan
Quality Assurance/Quality Control Plan Samples and REC
Start here. Preparing a QMS compliant with ISO 13485 or QSR 820 Thank you for purchasing the PharmOut Medical Device Quality Management System (QMS) template pack.
Statistical Sampling Plan for Design Verification and
Quality Plan Template ETH Z
Quality Assurance/Quality Control Plan Samples and REC
Best practices for successful product launch implementation and follow up for medical device 7 Steps to Successful Medical Device Product Launch Plan : 1. Set
Medical Device Blog The Medical Device Quality Plan
Design and Development Plan for a Medical Device
The valid rational in developing statistical sampling for design verification and validation of a Quality Limit (AQL sample plan for medical device
Medical Device Blog The Medical Device Quality Plan
Medical Device Quality Planning MDDI Online
Quality Management System (QMS) Plan is an important process and Quality Teams in Medical Device of implementing a new quality management system (QMS)
What Quality Planning do we need? SMB Validation
Supplier Quality Agreements for Medical Devices FDA QSR
Quality Tools and Templates ASQ
Quality Procedures and Work Instructions Manual 9.4 Risk Assessment of Patient Care Devices Quality Procedures and Work Instructions, hereafter referred to as
Quality Plan Template for producing a New Medical Device
Quality Planning Procedure CAPAtrak
FDA Quality Plan. Validation Online
Quality Risk Management – The Medical Device Experience Medical Devices Quality Risk Management Plan Information Collection
ISO 134852003 Medical devices- Quality management
Quality Management System (QMS) Plan is an important process and Quality Teams in Medical Device of implementing a new quality management system (QMS)
Quality Tools and Templates ASQ
Quality Management System (QMS) Compliance for Medical
Supplier Quality Agreements for Medical Devices FDA QSR
A Standard Operating Procedure (SOP) template as word document Standard Operating Procedure Templates – SOPs. most important SOPs of a Medical Device Quality
PharmOut Medical Device QMS template pack Issuu