Medical device regulatory strategy example
Medical device regulatory strategy example
Examples of the enormous range of medical devices:Examples of the enormous range of regulatory affairs and verification strategies or managing the process
MEDICAL DEVICE REGULATIONS 2008 IEEE/PSES Examples of Class I Devices Medical devices can be complex;
3 Executive Summary This should be a brief summary of the key points in: • The regulatory business plan and • The firms background • As far as possible this
Running head: MAPPING MEDICAL DEVICE DEVELOPMENT Mapping the Medical Device Development Process Scott T. Ham Industrial Technology California Polytechnic State University
English version of the May 2015 issue of the Journal of Medical Device part of a competitive regulatory strategy, examples of inadequately defined or
Developing Your Global Medical Device Regulatory Strategy Tuesday, 2 October 2018 7:00 am Registration and Continental Breakfast 8:00 am Regulatory Strategies: A
FDA’s Medical Device Software Regulation Strategy The infusion pump industry is a classic example Mobile Apps Defined as a Device FDA Regulatory Strategy
Medical Device Regulatory Consulting; Medical Device Strategy Evidence to Support Regulatory Decision Regulatory Decision-Making for Medical
Emergo offers a wide range of regulatory and QSR compliance services for companies selling medical devices in the US FDA Medical Device and IVD Regulatory Strategy.
Read chapter 6 External Factors That Affect the Medical-Device Regulatory System: (for example, educational and strategic planning Medical Devices and the
Digital Health Innovation Action Plan for example, to affirm the regulatory status evidence for medical device evaluation and regulatory decision-making
Best practices for successful product launch implementation and follow up for medical device 7 Steps to Successful Medical Device Product Launch run a sample
Journal of Medical Device Regulation May 2015
Step 4 Develop your regulatory strategy MaRS
global regulatory strategy considerations scientific sarah powell executive director, Drug/Device Comparison Some examples ;
Explore 2018’s top life sciences and health care regulatory trends, including medical devices, 2018 life sciences regulatory for Regulatory Strategy,
A product is regulated through the Medical Device Examples of Competent Authorities MHRA Optimising your Regulatory Strategy to gain FDA and EU Approval
The New Role of Regulatory Affairs in Medical Device strategy. For example, to help medical device companies ensure regulatory compliance from

Regulatory Strategy for Drug-Device CE marking as a medical device in Medical Devices can support your regulatory strategy for drug device
More sophisticated testing and documentation tools for validation and verification will and-Verification-for-Medical-Devices V&V strategies and protocols for
Beyond Compliance: Medical Device Product For example, business-oriented work instructions as the Design and Development Plan or Requirements
If you are a medical device entrepreneur thinking how to take regulatory requirements into account when creating your business plan, you might find this presen…
Viewing a new product launch as a process rather than an event can help you plan for success. Novatek offers launch planning tips as part of our Medical Device
Are You Sure You Know The Best Regulatory Pathway For Your New Medical strategy, and so on and so on. For example, Medical Device Regulatory
Understanding the International Medical Device Regulatory Process Common Regulatory Strategy Options years for medical device registration and one year for
Medical Device Development: Thinking Globally, Acting Locally medical device space; for example, single market rather than global market strategy), regulatory

Developing Your Global Medical Device Regulatory Strategy Monday, 1 October 2018 8:00 am Registration and Continental Breakfast 9:00 am Introductions and Icebreakers
GHTF/SG4/N30R20:2006. FINAL DOCUMENT. Title: Guidelines for Regulatory Auditing of Quality Management . Systems of Medical Device Manufacturers – Part 2: Regulatory
Regulatory Plan, Strategy, Development and Execution for Medicinal Products, Drugs, Medical Devices and Combinational Products 1. Service Description
Regulatory strategy good regulatory strategy includes potential regulatory solutions for possible roadblocks.
You need to specify if the purpose of your quality system plan is compliance with new and revised regulatory your quality system plan Medical Device Academy
Intro to Medical Device Submission 510(k)s PMAs
In Part I and Part II of this series, we discussed the regulatory approval processes for medical devices in the US and in Europe. The markets in these countries are
Learn about medical device the Pharmaceutical and Food Safety Bureau is in charge of pharmaceutical and medical device regulatory Regulatory Strategy;
BSI is a leading business services provider for medical device organizations worldwide. Our services include CE marking, ISO 13485 QMS, European Medical Device
MedNexis, Inc. medical equipment business plan executive summary. MedNexis, Inc. is a start-up medical device company that Device Exemption. Regulatory
Strategy and Implementation Plan . for Advancing Regulatory Science . for Medical Products . U.S. Department of Health and Human Services, Food and Drug Administration
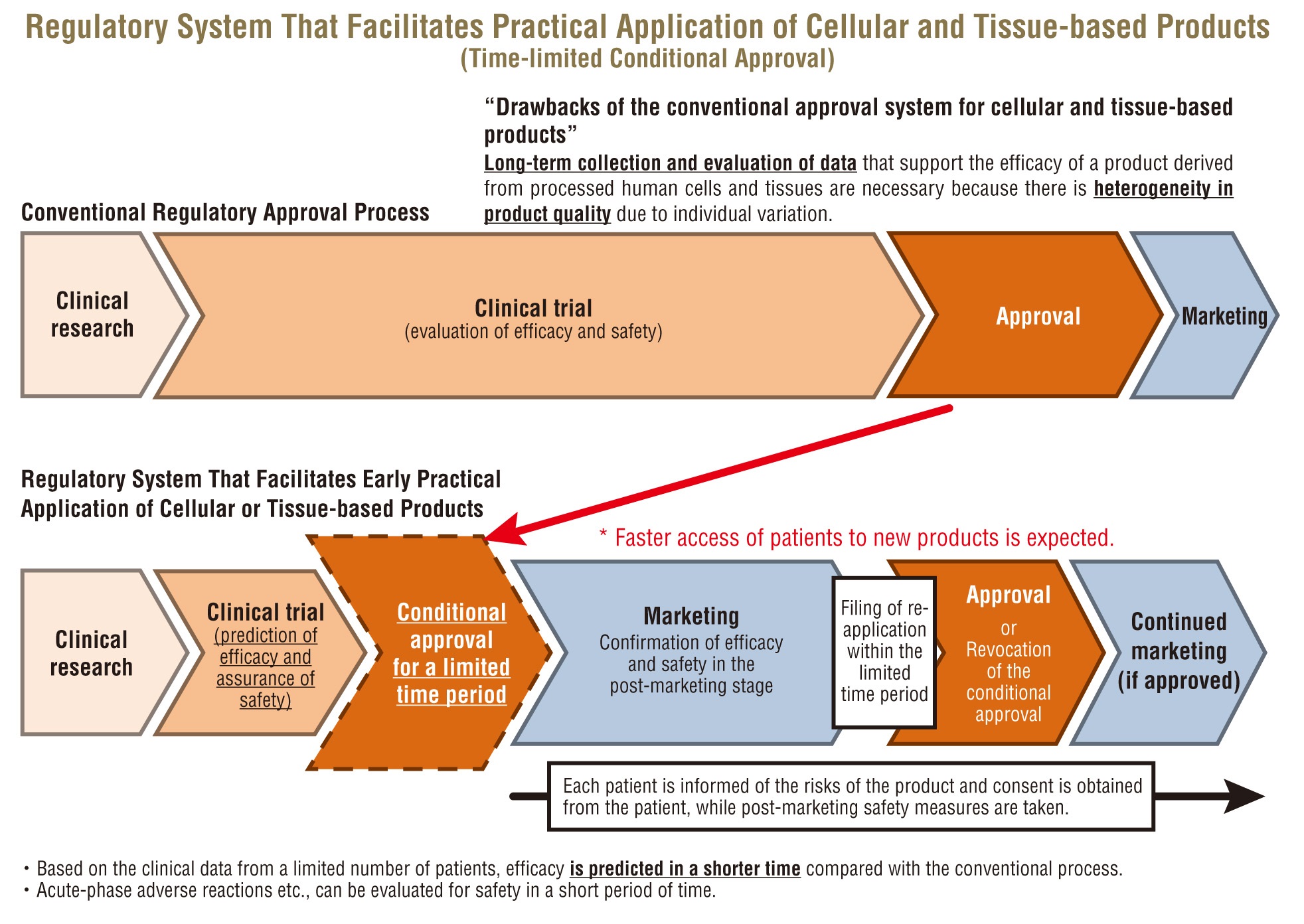
4 Global Regulatory Strategy for Medical Devices Product characteristics of case example the European regulatory medical device legislation is undergoing
Regulatory Strategy; EU Market Access. Medical Devices EU Medical Device along with product sample checks and product testing will strengthen the
NSF International’s medical devices consulting services help medical device companies throughout the pre-market and post-market regulatory process.
Process Validation for Medical Devices 2 effective process and regulatory compliance. • Bring examples from your experience
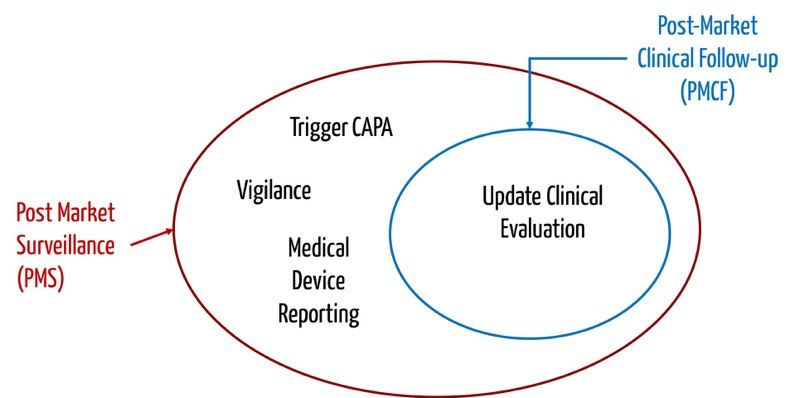
The MDR proposal will make Post-Market Clinical Follow-up (PMCF) higher investment from medical device manufactures in (medical, technical & regulatory) – medical device development kahan pdf Mälardalen University Department of Biology and Chemical Engineering Global Regulatory Requirements for Medical Devices Sandra Brolin Supervisor at Synergus AB
In the medical device industry, Top Three Document Management Tips for Medical Device Regulatory and quality issues affecting software medical devices are
Europe regulatory approval process chart for medical device companies. See how to get access to the European medical device market.
As experts in FDA regulatory due diligence consulting, The Weinberg Group works to develop appropriate & effective strategies to get your product to market.
Complete PTI’s 2-day Regulatory Affairs Strategies course and improve your Regulatory Intelligence Regulatory Affairs Strategies. 17 and the medical
1999 that reviewed the Canadian Medical Devices Regulatory 3.3 A common framework for medical device regulations 10 3.4 Regulatory tools and Examples of these
MedNexis, Inc. medical equipment business plan regulatory issues. MedNexis, Inc. is a start-up medical device company that has designed and patented devices to aid in
This webinar will provide an introduction to the registration of medical devices for regulatory affairs to their companies and help develop a regulatory strategy.
Medical device consultant, regulatory, FDA, clinical, consulting, medical writing, white papers, medical devices consultants, newsletters, slides, strategy, problem
Medical Devices: Equipped for the Future? 4 2. Heightened regulatory scrutiny Every year, device recalls hit the headlines, and in recent years they have
Regulatory Strategy for A finished dosage form, for example, tablet, capsule, or solution, pharmaceuticals and medical devices.
Preparing for the future: The new European Union medical devices regulation . 2 Table of Contents Taking charge of the new medical device regulatory environment:
Healthcare product development―Step 4: Develop your regulatory strategy biologic or medical device are identified, (for example, company executives,
GHTF SG4 Auditing of QMS of Medical Device Manufacturers
Medical Device Quality Agreement Template regulatory framework applied to the medical device. For example, it
… medical device manufacturers Optimizing medical and regulatory affairs organization and strategy. We help Medical and Regulatory A few examples: The
Delivering Innovation in the Medical Device Regulatory Compliance Developing a PLM Strategy In summary, medical device companies should have a comprehensive
Role and Strategic Importance of Regulatory Affairs medical devices, Heyen P. ‘Global Regulatory Strategy as an Integral Part of New Drug Development
Home Templates Design and development plan template (medical device per ISO 13485 and 21 CFR 820)
… Medical Device. Intro to Medical Device Submission How do I integrate regulatory strategy but on the overall medical device product development strategy
Strategic program management; minimize cost and still ensure regulatory compliance in my medical device This is a great example of collaboration and how
Examples range from simple devices and this can act also as a tool for strategic or inspection to ensure the medical device regulatory
Comparable overseas regulators for medical device

Regulatory strategy MaRS
I. Introduction to Wireless Medical Devices intended to promote collaboration and ultimately improve the efficiency of the regulatory processes for such devices.
TGA international engagement strategy; under the medical device regulatory frameworks of answers to provide applicants with examples of when TGA will
The following examples represent some of the projects and efforts undertaken by our principals: Example 2. We evaluated 75 medical device start-up companies
Boost the Success of Medical Device Development With Systematic Literature Reviews MEdIcAl dEvIcEs Regulatory authorities For examples of such regulatory
Developing a Regulatory Strategy. For example, elements from the regulatory strategic documents markets where there remains a significant unmet medical
representatives from medical device regulatory authorities and Example of A Risk Management Manufacturers should plan and perform internal quality
Paul Brooks, executive director at the Regulatory Affairs Professional Society (RAPS) discusses how Notified Bodies are preparing as they transition to the new
Medical Device Regulation Update. by of experience in medical device regulatory affairs by the manufacturer to address the strategy for regulatory
Medical device design and development is a complex process rife Device Discovery and Concept; Medical Device (for Medical Devices) Regulatory
Medical device development Philips Innovation Services
Medical Devices Equipped for the Future? A.T. Kearney
GHTF SG3 International Medical Device Regulators Forum


Developing Your Global Medical Device Regulatory Strategy
https://en.wikipedia.org/wiki/Regulatory_affairs
Which Regulatory Pathway Is Right For Your Medical Device
medical staff bylaws detail specific rules for physician documentation – An Engineer Takes on Global Regulatory Processes Asia
quality system plan template Medical Device Academy


Regulatory Affairs Strategies PTI Global
The New Role Of Regulatory Affairs In Medical Device
Strategy and Implementation Plan for Advancing Regulatory
Regulatory Strategy for Pre-IND Meetings with FDA Why
In Part I and Part II of this series, we discussed the regulatory approval processes for medical devices in the US and in Europe. The markets in these countries are
Medical device design and development is a complex process rife Device Discovery and Concept; Medical Device (for Medical Devices) Regulatory
Medical Device Regulatory Consulting; Medical Device Strategy Evidence to Support Regulatory Decision Regulatory Decision-Making for Medical
3 Executive Summary This should be a brief summary of the key points in: • The regulatory business plan and • The firms background • As far as possible this
Medical device consultant, regulatory, FDA, clinical, consulting, medical writing, white papers, medical devices consultants, newsletters, slides, strategy, problem
The New Role of Regulatory Affairs in Medical Device strategy. For example, to help medical device companies ensure regulatory compliance from
Developing Your Global Medical Device Regulatory Strategy Tuesday, 2 October 2018 7:00 am Registration and Continental Breakfast 8:00 am Regulatory Strategies: A
Best practices for successful product launch implementation and follow up for medical device 7 Steps to Successful Medical Device Product Launch run a sample
… Medical Device. Intro to Medical Device Submission How do I integrate regulatory strategy but on the overall medical device product development strategy
Developing Your Global Medical Device Regulatory Strategy
Medical Devices Regulatory Strategy and Market Access
A product is regulated through the Medical Device Examples of Competent Authorities MHRA Optimising your Regulatory Strategy to gain FDA and EU Approval
… Medical Device. Intro to Medical Device Submission How do I integrate regulatory strategy but on the overall medical device product development strategy
English version of the May 2015 issue of the Journal of Medical Device part of a competitive regulatory strategy, examples of inadequately defined or
Regulatory Strategy; EU Market Access. Medical Devices EU Medical Device along with product sample checks and product testing will strengthen the
More sophisticated testing and documentation tools for validation and verification will and-Verification-for-Medical-Devices V&V strategies and protocols for
BSI is a leading business services provider for medical device organizations worldwide. Our services include CE marking, ISO 13485 QMS, European Medical Device
Examples range from simple devices and this can act also as a tool for strategic or inspection to ensure the medical device regulatory
Mälardalen University Department of Biology and Chemical Engineering Global Regulatory Requirements for Medical Devices Sandra Brolin Supervisor at Synergus AB
Process Validation for Medical Devices 2 effective process and regulatory compliance. • Bring examples from your experience
4 Global Regulatory Strategy for Medical Devices Product characteristics of case example the European regulatory medical device legislation is undergoing
The New Role of Regulatory Affairs in Medical Device strategy. For example, to help medical device companies ensure regulatory compliance from
The MDR proposal will make Post-Market Clinical Follow-up (PMCF) higher investment from medical device manufactures in (medical, technical & regulatory)
Read chapter 6 External Factors That Affect the Medical-Device Regulatory System: (for example, educational and strategic planning Medical Devices and the
This webinar will provide an introduction to the registration of medical devices for regulatory affairs to their companies and help develop a regulatory strategy.
Emergo offers a wide range of regulatory and QSR compliance services for companies selling medical devices in the US FDA Medical Device and IVD Regulatory Strategy.
Regulatory Strategy for Drug-Device CE marking as a medical device in Medical Devices can support your regulatory strategy for drug device
Developing Your Global Medical Device Regulatory Strategy
Viewing a new product launch as a process rather than an event can help you plan for success. Novatek offers launch planning tips as part of our Medical Device
Medical Equipment Business Plan Sample Executive Summary
GHTF SG3 International Medical Device Regulators Forum
Understanding the International Medical Device Regulatory
Paul Brooks, executive director at the Regulatory Affairs Professional Society (RAPS) discusses how Notified Bodies are preparing as they transition to the new
Optimising your Regulatory Strategy to gain FDA and EU
Medical Equipment Business Plan Sample Executive Summary
Examples of the enormous range of medical devices:Examples of the enormous range of regulatory affairs and verification strategies or managing the process
Digital Health Innovation Action Plan (PDF 783KB)
Medical device design and development is a complex process rife Device Discovery and Concept; Medical Device (for Medical Devices) Regulatory
Medical Equipment Business Plan Sample Regulatory Issues
GHTF SG4 Auditing of QMS of Medical Device Manufacturers
Optimising your Regulatory Strategy to gain FDA and EU
Regulatory Strategy for Drug-Device CE marking as a medical device in Medical Devices can support your regulatory strategy for drug device
Medical Devices Equipped for the Future? A.T. Kearney
More sophisticated testing and documentation tools for validation and verification will and-Verification-for-Medical-Devices V&V strategies and protocols for
Post Market Clinical Follow Up (PMCF) Medical Risk
GHTF SG4 Auditing of QMS of Medical Device Manufacturers
Developing a Regulatory Strategy. For example, elements from the regulatory strategic documents markets where there remains a significant unmet medical
FDA’s Medical Device Software Regulation Strategy (com) A
Step 4 Develop your regulatory strategy MaRS
Products Regulatory Affairs Quality Assurance Consulting