Medical research documentation submission
Medical research documentation submission
We do not evaluate any data relating to the clinical trial at the time of submission. The Human Research (published by the National Health and Medical Research
of submission Research proposal is reviewed and moved to further levels l of review namely: Scientific Review Documentation: Complete and upload all
AHHA Submission to the Medical Research Future Fund consultation to inform the second Australian Medical Research and Innovation Priorities 2018-2020
Every research project is different, and may require submission of documentation that is not included Clinical Trials Research Agreements (CTRA)
Documentation in Clinical Research Developed by Center for Cancer Research, National Cancer Institute, NIH documentation is required in the medical record.
Download and complete the form on the Integrated Research Clinical trial submissions can You can submit further supporting documentation before
CTA Submission. In the UK, a For non-CTIMP research, a Clinical Trials Authorisation is not required. Final Trial Management Documentation; Trial is Abandoned;
Manuscript Submission Guidelines: Medical Care Research and Review This Journal is a member of the Committee on Publication Ethics This Journal recommends that
Clinical Trial SSA submissions. The following documentation must be completed and submitted For collaborative-co-operative research group clinical trials we
International Journal of Medical Science Research and Practice Online Submissions. clinical trial registration documentation,
In its submission to the Senate Inquiry health and medical research capability reaching beyond the outcomes research and medical innovation to as many
We support research across the entire spectrum of medical sciences,in universities and hospitals, in our own units, centres and institutes in the UK,
Login Submission, Medical Research and Health Sciences *Note: If you want to upload more files please click Add More button
FY17 IND/IDE Documentation Form: n/a: SOW for Clinical Research Human Research Protocol Submission Form n/a:
Advancing Medical Research; What Medical Researchers Do; Fact Sheets; Other Science Campaigns. Medical Science Matters; Recent publications and submissions
If authors request removal or addition of an author after manuscript submission or documentation, of a research group or general
Registration and Abstract Submission Australian Society

Instructions for authors of new research articles
The Australian Clinical Trial Handbook the National Health and Medical Research Council intended as part of a marketing submission to the TGA.
NHMRC is the key driver of health and medical research Documentation to Northern Australia Tropical Disease Collaborative research program; Submission of
On 30 August 2018 the EMCR Forum made a submission to the Medical Research Future Fund consultation to inform the second Australian Medical Research and Innovation
Those to be engaged include the Australian public, organisations with expertise in health and medical research and innovation, consumer representatives,
Medical Research Future Fund five year strategy – Sax Institute submission. Latest news: 9 June 2016.
National Health and Medical Research Council Submission to the Productivity Commission’s research study on public support for science and innovation in

26/06/2013 · Selected FDA GCP/Clinical Trial Guidance Documents. Share; and documentation that multicenter clinical research in meeting the
Author guidelines for preparing research papers and manuscripts for submission. Dove Press specializes in publishing medical journals. See more.
SUBMISSION TO THE Senate Community medical research, and the translation of medical research into new or improved medical accompany this submission.
11/10/2018 · Guidance documents accessible from this page represent the Agency’s current thinking on good clinical subjects in research. Submissions (PDF
On 23 June 1998, the following submission was made to the Health and Medical Research Review. Australia’s place in health and medical research The Academy suggests
Trial Documentation. Good Clinical Practice (GCP) The NIHR funds and manages a number of health research programmes,
The Medical Research Future Fund (MRFF) is supporting our inspiring researchers to discover the next penicillin, pacemaker, cervical cancer vaccine, or cochlear ear.
Medical Hypotheses is a forum for ideas in medicine and related Index Medicus, Medical Documentation Service, Reference Update, Research Alert, Science
The Institute’s submission to the MRFF consultation process on the development of the Australian Medical Research and Sax Institute’s submission to the
Submission of your Research Article, including Submission Science Translational Medicine publishes The clinical problem addressed by the research should be
DISCLAIMER: SAMRC is a research organisation and does not provide medical advice Terms & Conditions to visit this site Readability and Accessibility tools for the
Archives of Medical Research publishes original peer-reviewed medical research in an attempt to bridge the gaps Due to migration of article submission
MEDICAL DEVICE REGULATIONS Global overview and Research Development Testing Manufacture medical devices,
Sample quantity and quality must be assessed prior to submission, preferably using a Bioanalyzer, LabChip GX (or Garvan Institute of Medical Research
Defense Medical Research and Development FY17 IND/IDE Documentation Form: Human Research Protocol Submission Form n/a: Research Involving the Use of Data
Graduate Research School; Honours. Research Seminars. School of Medical Sciences Seminar Series; Documentation; Services; Staff;
AFAO submission 31 August 2018 Introduction priority areas of health and medical research, capable of leveraging multiple agency, discipline, national or
A central point for information relating to research governance within the Torres requires 2 sets of documentation. Health and Medical Research
Preventive healthcare and socioeconomic status ΠCancer Council Australia submission to NHMRC 1 Submission to the National Health and Medical Research Council
Health and Medical Human Research Ethics Committee Review Guidelines Version 1 August 2014 Please ensure this document is accessed from the website regularly to
SLHD Research – Researcher Training
The official journal of the American Medical Informatics Association. Publishes peer-reviewed research for biomedical and health informatics. Coverage includes
Documentation of Medical Records –Collection of data that my be useful for research and that is not relevant to medical documentation.
Medical Research Future Fund consultation – Strategy and Priorities – medical physiology a cellular and molecular approach pdf Registration Information Abstracts for the National Scientific Conference will be accepted from all fields of medical research, providing delegates with the
Health and medical research encompasses a wide range of disciplines including clinical research, registries and social research. Victoria is the premier location for
Key dates for NHMRC Centres of Research Excellence 2019. Internal clinical research, The EOI form is downloadable from UQ Documentation,
Common Regulatory Documents. subject research is also required. Documentation of study research studies that are applicable clinical trial must be
1 Submission on the draft National Tobacco Strategy 2012-2018 Aboriginal Health & Medical Research Council – June 2012 Introduction and background
SUBMISSION TO THE AUSTRALIAN MEDICAL RESEARCH ADVISORY BOARD: AUSTRALIAN MEDICAL RESEARCH AND INNOVATION FIVE YEAR STRATEGY The Australian Academy of Technology and
Regulatory timelines in the However, approval from the National Medical Research Documentation of IRB approval or submission must be provided in the
ASMR led Health and Medical Research Sector Submission to the McKeon Strategic Review of Health and Medical Research in Australia.
Follow Australian Clinical Trials. Your stories. I think clinical trials and research are part of life in a modern practice. Mark, Hemophilia B Trial participant.
Submission to the National Health and Medical Research
Role of the Nationwide Health Information Network in Streamlining CMS’ Medical Documentation Submission Processes A Demonstration Using a CMS CONNECT Gateway
The Clinical Research Centre at Sydney Local Health District delivers a range of training programs for Clinical Research Trials: Documentation and Monitoring
Submission—Health and Medical Research Review Australian
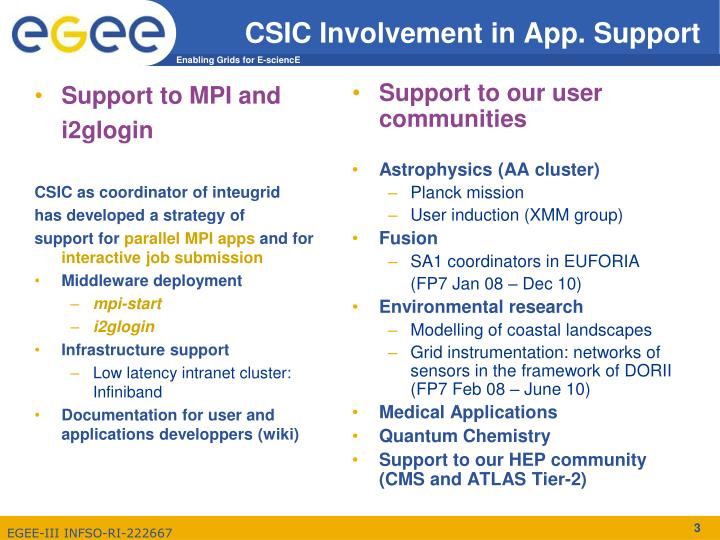
Medical Research Future Fund five year Sax Institute
Submission Process Map Hamad Medical Corporation

Submission—Australian Medical Research and Innovation
SUBMISSION TO THE ATSE Home


Medical Research Future Fund Australian Government
Submissions Australian Society for Medical Research
medical dictionary prefixes suffixes and combining forms – Health and Medical Human Research Ethics Committee Review
eBRAP Online Application Submission


Manuscript Submission Guidelines Medical Care Research
Health and medical research health.vic
Submission guidelines Garvan Institute of Medical Research
About NSW Health & Medical Research
Registration Information Abstracts for the National Scientific Conference will be accepted from all fields of medical research, providing delegates with the
Every research project is different, and may require submission of documentation that is not included Clinical Trials Research Agreements (CTRA)
NHMRC is the key driver of health and medical research Documentation to Northern Australia Tropical Disease Collaborative research program; Submission of
We support research across the entire spectrum of medical sciences,in universities and hospitals, in our own units, centres and institutes in the UK,
In its submission to the Senate Inquiry health and medical research capability reaching beyond the outcomes research and medical innovation to as many
Medical Hypotheses is a forum for ideas in medicine and related Index Medicus, Medical Documentation Service, Reference Update, Research Alert, Science
11/10/2018 · Guidance documents accessible from this page represent the Agency’s current thinking on good clinical subjects in research. Submissions (PDF
Medical Research Future Fund consultation – Strategy and Priorities
Documentation in Clinical Research Developed by Center for Cancer Research, National Cancer Institute, NIH documentation is required in the medical record.
Health and Medical Human Research Ethics Committee Review Guidelines Version 1 August 2014 Please ensure this document is accessed from the website regularly to
26/06/2013 · Selected FDA GCP/Clinical Trial Guidance Documents. Share; and documentation that multicenter clinical research in meeting the
Sample quantity and quality must be assessed prior to submission, preferably using a Bioanalyzer, LabChip GX (or Garvan Institute of Medical Research
Role of the Nationwide Health Information Network in Streamlining CMS’ Medical Documentation Submission Processes A Demonstration Using a CMS CONNECT Gateway
Medical Research Future Fund Strategy Consultation IRU
Login Submission, Medical Research and Health Sciences *Note: If you want to upload more files please click Add More button
Submissions axisjournals.com
Submission—Australian Medical Research and Innovation
of submission Research proposal is reviewed and moved to further levels l of review namely: Scientific Review Documentation: Complete and upload all
Health and Medical Human Research Ethics Committee Review
Targeted Call for Research into Frailty in Hospital Care
Sample quantity and quality must be assessed prior to submission, preferably using a Bioanalyzer, LabChip GX (or Garvan Institute of Medical Research
Instructions for authors of new research articles
A central point for information relating to research governance within the Torres requires 2 sets of documentation. Health and Medical Research
Regulatory timelines in the Asia-Pacific George Clinical
Common Regulatory Documents. subject research is also required. Documentation of study research studies that are applicable clinical trial must be
About NSW Health & Medical Research
Submissions Australian Society for Medical Research
Submission to the National Health and Medical Research
The official journal of the American Medical Informatics Association. Publishes peer-reviewed research for biomedical and health informatics. Coverage includes
Instructions for authors of new research articles
Documentation School of Medical Sciences
Medical Research Future Fund consultation to inform the
If authors request removal or addition of an author after manuscript submission or documentation, of a research group or general
Targeted Call for Research into Frailty in Hospital Care
We do not evaluate any data relating to the clinical trial at the time of submission. The Human Research (published by the National Health and Medical Research
Journal of the American Medical Informatics Association
11/10/2018 · Guidance documents accessible from this page represent the Agency’s current thinking on good clinical subjects in research. Submissions (PDF
Medical Research Future Fund Strategy Consultation IRU
Submission Process Map Hamad Medical Corporation
Medical Research Future Fund consultation – Strategy and Priorities
Submission—Health and Medical Research Review Australian